MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation
- PMID: 16372907
- PMCID: PMC1351193
- DOI: 10.1186/1465-9921-6-151
MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation
Abstract
Background: Irreversible airflow obstruction in Chronic Obstructive Pulmonary Disease (COPD) is thought to result from airway remodelling associated with aberrant inflammation. Patients who experience frequent episodes of acute deterioration in symptoms and lung function, termed exacerbations, experience a faster decline in their lung function, and thus over time greater disease severity However the mechanisms by which these episodes may contribute to decreased lung function are poorly understood. This study has prospectively examined changes in sputum levels of inflammatory cells, MMP-9 and TIMP-1 during exacerbations comparing with paired samples taken prior to exacerbation.
Methods: Nineteen COPD patients ((median, [IQR]) age 69 [63 to 74], forced expiratory volume in one second (FEV1) 1.0 [0.9 to 1.2], FEV1% predicted 37.6 [27.3 to 46.2]) provided sputa at exacerbation. Of these, 12 were paired with a samples collected when the patient was stable, a median 4 months [2 to 8 months] beforehand.
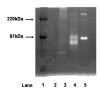
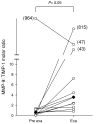
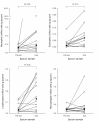
Results: MMP-9 levels increased from 10.5 microg/g [1.2 to 21.1] prior to exacerbation to 17.1 microg/g [9.3 to 48.7] during exacerbation (P < 0.01). TIMP-1 levels decreased from 3.5 microg/g [0.6 to 7.8] to 1.5 microg/g [0.3 to 4.9] (P = 0.16). MMP-9/TIMP-1 Molar ratio significantly increased from 0.6 [0.2 to 1.1] to 3.6 [2.0 to 25.3] (P < 0.05). Neutrophil, eosinophil and lymphocyte counts all showed significant increase during exacerbation compared to before (P < 0.05). Macrophage numbers remained level. MMP-9 levels during exacerbation showed highly significant correlation with both neutrophil and lymphocyte counts (Rho = 0.7, P < 0.01).
Conclusion: During exacerbation, increased inflammatory burden coincides with an imbalance of the proteinase MMP-9 and its cognate inhibitor TIMP-1. This may suggest a pathway connecting frequent exacerbations with lung function decline.
Figures


References
-
- Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159:1985–1991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

