Targeting the active site of the placental isozyme of alkaline phosphatase by phage-displayed scFv antibodies selected by a specific uncompetitive inhibitor
- PMID: 16372914
- PMCID: PMC1351172
- DOI: 10.1186/1472-6750-5-33
Targeting the active site of the placental isozyme of alkaline phosphatase by phage-displayed scFv antibodies selected by a specific uncompetitive inhibitor
Abstract
Background: The isozymes of alkaline phosphatase, the tissue non-specific, intestinal and placental, have similar properties and a high degree of identity. The placental isozyme (PLAP) is an oncofetal antigen expressed in several malignancies including choriocarcinoma, seminoma and ovarian carcinoma. We had earlier attempted to isolate PLAP-specific scFv from a synthetic human immunoglobulin library but were unable to do so, presumably because of the similarity between the isozymes. In this work, we have employed a PLAP-specific uncompetitive inhibitor, L-Phe-Gly-Gly, to select isozyme specific scFvs. An uncompetitive inhibitor binds to the enzyme in the presence of substrate and stabilizes the enzyme-substrate complex. Several uncompetitive inhibitors have varying degrees of isozyme specificity for human alkaline phosphatase isozymes. A specific uncompetitive inhibitor would be able to unmask conformational differences between the otherwise very similar molecules. Also, such inhibitors would be directed to regions at/close to the active site of the enzyme. In this work, the library was first incubated with PLAP and the bound clones then eluted by incubation with L-Phe-Gly-Gly along with the substrate, para-nitro phenyl phosphate (pNPP). The scFvs were then studied with regard to the biochemical modulation of their binding, isozyme specificity and effect on enzyme activity.
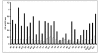
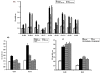
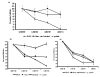
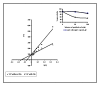
Results: Of 13 clones studied initially, the binding of 9 was inhibited by L-Phe-Gly-Gly (with pNPP) and 2 clones were inhibited by pNPP alone. Two clones had absolute and 2 clones had partial specificity to PLAP. Two clones were cross-reactive with only one other isozyme. Three scFv clones, having an accessible His6-tag, were purified and studied for their modulation of enzyme activity. All the three scFvs inhibited PLAP activity with the kinetics of competitive inhibition. Cell ELISA could demonstrate binding of the specific scFvs to the cell surface expressed PLAP.
Conclusion: The results demonstrate the biochemical modulation of scFv binding. Also, the scFvs bound to the active site and denied the access to the substrate. The selection strategy could generate specific anti-enzyme antibodies to PLAP that can potentially be used for targeting, for modulating enzyme activity in in vitro and in vivo and as probes for the active site. This strategy also has a general application in selecting antibodies from combinatorial libraries to closely related molecules and conformations.
Figures




Similar articles
-
A phage antibody to the active site of human placental alkaline phosphatase with higher affinity to the enzyme-substrate complex.Mol Immunol. 2007 Jan;44(4):369-76. doi: 10.1016/j.molimm.2006.02.024. Epub 2006 Apr 4. Mol Immunol. 2007. PMID: 16600380
-
Molecular mechanism of uncompetitive inhibition of human placental and germ-cell alkaline phosphatase.Biochem J. 1992 Aug 15;286 ( Pt 1)(Pt 1):23-30. doi: 10.1042/bj2860023. Biochem J. 1992. PMID: 1520273 Free PMC article.
-
Evidence for homology of normal and neoplastic human placental alkaline phosphatases as determined by monoclonal antibodies to the cancer-associated enzyme.Cancer Res. 1985 Jul;45(7):3268-73. Cancer Res. 1985. PMID: 2408748
-
Clinical and biological significance of an isozyme tumor marker--PLAP.Clin Biochem. 1987 Dec;20(6):387-92. doi: 10.1016/0009-9120(87)90003-8. Clin Biochem. 1987. PMID: 3325192 Review.
-
Placental alkaline phosphatase as the placental IgG receptor.Clin Chem. 1992 Dec;38(12):2543-5. Clin Chem. 1992. PMID: 1458596 Review.
Cited by
-
Identification of a New Uncompetitive Inhibitor of Adenosine Deaminase from Endophyte Aspergillus niger sp.Curr Microbiol. 2018 May;75(5):565-573. doi: 10.1007/s00284-017-1418-4. Epub 2017 Dec 14. Curr Microbiol. 2018. PMID: 29243069
-
A comparative study of the lateral geniculate body of rat (Rattus norvegicus), bat (Eidolon helvum) and pangolin (Manis tricuspis).Glob J Health Sci. 2012 Jun 12;4(4):118-25. doi: 10.5539/gjhs.v4n4p118. Glob J Health Sci. 2012. PMID: 22980348 Free PMC article.
-
Recombinant antibodies for specific detection of clostridial [Fe-Fe] hydrogenases.Sci Rep. 2016 Oct 27;6:36034. doi: 10.1038/srep36034. Sci Rep. 2016. PMID: 27786270 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

