ATP-sensitive K+ channels: regulation of bursting by the sulphonylurea receptor, PIP2 and regions of Kir6.2
- PMID: 16373383
- PMCID: PMC1796795
- DOI: 10.1113/jphysiol.2005.100719
ATP-sensitive K+ channels: regulation of bursting by the sulphonylurea receptor, PIP2 and regions of Kir6.2
Abstract
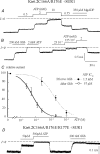
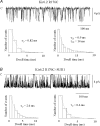
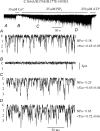
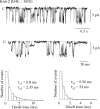
ATP-sensitive K+ channels composed of the pore-forming protein Kir6.2 and the sulphonylurea receptor SUR1 are inhibited by ATP and activated by Phosphatidylinositol Bisphosphate (PIP2). Residues involved in binding of these ligands to the Kir6.2 cytoplasmic domain have been identified, and it has been hypothesized that gating mechanisms involve conformational changes in the regions of the bundle crossing and/or the selectivity filter of Kir6.2. Regulation of Kir6.2 by SUR1, however, is not well-understood, even though this process is ATP and PIP2 dependent. In this study, we investigated the relationship between channel regulation by SUR1 and PIP2 by comparing a number of single and double mutants known to affect open probability (P(o)), PIP2 affinity, and sulphonylurea and MgADP sensitivity. When coexpressed with SUR1, the Kir6.2 mutant C166A, which is characterized by a P(o) value close to 0.8, exhibits no sulphonylurea or MgADP sensitivity. However, when P(o) was reduced by combining mutations at the PIP2-sensitive residues R176 and R177 with C166A, sulphonylurea and MgADP sensitivities were restored. These effects correlated with a dramatic decrease in PIP2 affinity, as assessed by PIP2-induced channel reactivation and inhibition by neomycin, an antagonist of PIP2 binding. Based on macroscopic and single-channel data, we propose a model in which entry into the high-P(o) bursting state by the C166A mutation or by SUR1 depends on the interaction of PIP2 with R176 and R177 and, to a lesser extent, R54. In conjunction with this PIP2-dependent process, SUR1 also regulates channel activity via a PIP2-independent, but MgADP-dependent process.
Figures





References
-
- Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–41580. - PubMed
-
- Babenko AP, Gonzalez G, Bryan J. The N-terminus of KIR6.2 limits spontaneous bursting and modulates the ATP-inhibition of KATP channels. Biochem Biophys Res Commun. 1999;255:231–238. - PubMed
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

