Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones
- PMID: 16373387
- PMCID: PMC1796796
- DOI: 10.1113/jphysiol.2005.097006
Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones
Erratum in
- J Physiol. 2006 Apr 1;572(Pt 1):307
Abstract
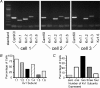
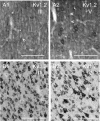
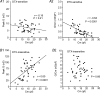
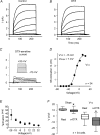
Potassium channels are extremely diverse regulators of neuronal excitability. As part of an investigation into how this molecular diversity is utilized by neurones, we examined the expression and biophysical properties of native Kv1 channels in layer II/III pyramidal neurones from somatosensory and motor cortex. Single-cell RT-PCR, immunocytochemistry, and whole cell recordings with specific peptide toxins revealed that individual pyramidal cells express multiple Kv1 alpha-subunits. The most abundant subunit mRNAs were Kv1.1 > 1.2 > 1.4 > 1.3. All of these subunits were localized to somatodendritic as well as axonal cell compartments. These data suggest variability in the subunit complexion of Kv1 channels in these cells. The alpha-dendrotoxin (alpha-DTX)-sensitive current activated more rapidly and at more negative potentials than the alpha-DTX-insensitive current, was first observed at voltages near action potential threshold, and was relatively insensitive to holding potential. The alpha-DTX-sensitive current comprised about 10% of outward current at steady-state, in response to steps from -70 mV. From -50 mV, this percentage increased to approximately 20%. All cells expressed an alpha-DTX-sensitive current with slow inactivation kinetics. In some cells a transient component was also present. Deactivation kinetics were voltage dependent, such that deactivation was slow at potentials traversed by interspike intervals during repetitive firing. Because of its kinetics and voltage dependence, the alpha-DTX-sensitive current should be most important at physiological resting potentials and in response to brief stimuli. Kv1 channels should also be important at voltages near threshold and corresponding to interspike intervals.
Figures




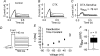

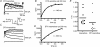
References
-
- Baldwin TJ, Tsaur M-L, Lopez GA, Jan YN, Jan LY. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

