Breaking symmetry in protein dimers: designs and functions
- PMID: 16373473
- PMCID: PMC2242361
- DOI: 10.1110/ps.051658406
Breaking symmetry in protein dimers: designs and functions
Abstract
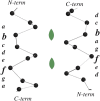
Symmetry, and in particular point group symmetry, is generally the rule for the global arrangement between subunits in homodimeric and other oligomeric proteins. The structures of fragments of tropomyosin and bovine fibrinogen are recently published examples, however, of asymmetric interactions between chemically identical chains. Their departures from strict twofold symmetry are based on simple and generalizable chemical designs, but were not anticipated prior to their structure determinations. The current review aims to improve our understanding of the structural principles and functional consequences of asymmetric interactions in proteins. Here, a survey of >100 diverse homodimers has focused on the structures immediately adjacent to the twofold axis. Five regular frameworks in alpha-helical coiled coils and antiparallel beta-sheets accommodate many of the twofold symmetric axes. On the basis of these frameworks, certain sequence motifs can break symmetry in geometrically defined manners. In antiparallel beta-sheets, these asymmetries include register slips between strands of repeating residues and the adoption of different side-chain rotamers to avoid steric clashes of bulky residues. In parallel coiled coils, an axial stagger between the alpha-helices is produced by clusters of core alanines. Such simple designs lead to a basic understanding of the functions of diverse proteins. These functions include regulation of muscle contraction by tropomyosin, blood clot formation by fibrin, half-of-site reactivity of caspase-9, and adaptive protein recognition in the matrix metalloproteinase MMP9. Moreover, asymmetry between chemically identical subunits, by producing multiple equally stable conformations, leads to unique dynamic and self-assembly properties.
Figures
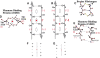
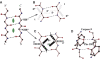
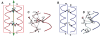
References
-
- Amor, J.C., Harrison, D.H., Kahn, R.A., and Ringe, D. 1994. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature 372: 704–708. - PubMed
-
- Anfinsen, C.B. 1973. Principles that govern the folding of protein chains. Science 181: 223–230. - PubMed
-
- Banner, D.W., Kokkinidis, M., and Tsernoglou, D. 1987. Structure of the ColE1 rop protein at 1.7 Å resolution. J. Mol. Biol. 196: 657–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

