Virus-like particles of the Ty3 retrotransposon assemble in association with P-body components
- PMID: 16373495
- PMCID: PMC1370889
- DOI: 10.1261/rna.2264806
Virus-like particles of the Ty3 retrotransposon assemble in association with P-body components
Abstract
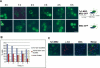
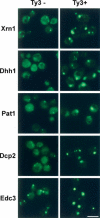
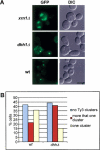
Retroviruses and retrotransposons assemble intracellular immature core particles around a RNA genome, and nascent particles collect in association with membranes or as intracellular clusters. How and where genomic RNA are identified for retrovirus and retrotransposon assembly, and how translation and assembly processes are coordinated is poorly understood. To understand this process, the subcellular localization of Ty3 RNA and capsid proteins and virus-like particles was investigated. We demonstrate that mRNAs, proteins, and virus-like particles of the yeast Ty3 retrotransposon accumulate in association with cytoplasmic P-bodies, which are sites of mRNA translation repression, storage, and degradation. Deletions of genes encoding P-body proteins decreased Ty3 transposition and caused changes in the pattern of Ty3 foci, underscoring the biological significance of the association of Ty3 virus-like protein components and P-bodies. These results suggest the hypothesis that P-bodies may serve to segregate translation and assembly functions of the Ty3 genomic RNA to promote assembly of virus-like particles. Because Ty3 has features of a simple retrovirus and P-body functions are conserved between yeast and metazoan organisms, these findings may provide insights into host factors that facilitate retrovirus assembly.
Figures



References
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. 1999. Current protocols in molecular biology. Greene Publishing Associates/Wiley-Interscience, New York.
-
- Basyuk, E., Galli, T., Mougel, M., Blanchard, J.M., Sitbon, M., and Bertrand, E. 2003. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell 5: 161–174. - PubMed
-
- Beach, D.L., Salmon, E.D., and Bloom, K. 1999. Localization and anchoring of mRNA in budding yeast. Curr. Biol. 9: 569–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
