p54nrb is a component of the snRNP-free U1A (SF-A) complex that promotes pre-mRNA cleavage during polyadenylation
- PMID: 16373496
- PMCID: PMC1370891
- DOI: 10.1261/rna.2213506
p54nrb is a component of the snRNP-free U1A (SF-A) complex that promotes pre-mRNA cleavage during polyadenylation
Abstract
The U1 snRNP-A (U1A) protein has been known for many years as a component of the U1 snRNP. We have previously described a form of U1A present in human cells in significant amounts that is not associated with the U1 snRNP or U1 RNA but instead is part of a novel complex of non-snRNP proteins that we have termed snRNP-free U1A, or SF-A. Antibodies that specifically recognize this complex inhibit in vitro splicing and polyadenylation of pre-mRNA, suggesting that this complex may play an important functional role in these mRNA-processing activities. This finding was underscored by the determination that one of the components of this complex is the polypyrimidine-tract-binding protein-associated splicing factor, PSF. In order to further our studies on this complex and to determine the rest of the components of the SF-A complex, we prepared several stable HeLa cell lines that overexpress a tandem-affinity-purification-tagged version of U1A (TAP-tagged U1A). Nuclear extract was prepared from one of these cell lines, line 107, and affinity purification was performed along with RNase treatment. We have used mass spectrometry analysis to identify the candidate factors that associate with U1A. We have now identified and characterized PSF, p54(nrb), and p68 as novel components of the SF-A complex. We have explored the function of this complex in RNA processing, specifically cleavage and polyadenylation, by performing immunodepletions followed by reconstitution experiments, and have found that p54(nrb) is critical.
Figures



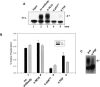
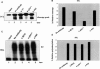

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
