Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production
- PMID: 16373502
- PMCID: PMC1324998
- DOI: 10.1073/pnas.0509459103
Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production
Abstract
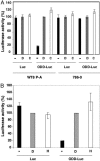
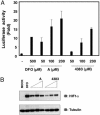
Many human diseases are characterized by the development of tissue hypoxia. Inadequate oxygenation can cause cellular dysfunction and death. Tissues use many strategies, including induction of angiogenesis and alterations in metabolism, to survive under hypoxic conditions. The heterodimeric transcription factor hypoxia-inducible factor (HIF) is a master regulator of genes that promote adaptation to hypoxia. HIF activity is linked to oxygen availability because members of the EGLN family hydroxylate HIFalpha subunits on specific prolyl residues when oxygen is present, which marks them for ubiquitination and proteasomal degradation. We created a mouse that ubiquitously expresses a bioluminescent reporter consisting of firefly luciferase fused to a region of HIF that is sufficient for oxygen-dependent degradation. Our validation studies suggest that this mouse will be useful for monitoring hypoxic tissues and evaluating therapeutic agents that stabilize HIF. One such agent, the HIF prolyl hydroxylase inhibitor FG-4383, was active in the liver and kidney after systemic administration as determined by bioluminescence imaging, transcription profiling, and production of erythropoietin, indicating that the HIF transcriptional program can be manipulated in vivo with orally active organic small molecules.
Figures

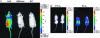

References
-
- Weissleder, R. & Ntziachristos, V. (2003) Nat. Med. 9, 123–128. - PubMed
-
- Gross, S. & Piwnica-Worms, D. (2005) Cancer Cell 7, 5–15. - PubMed
-
- Schofield, C. J. & Ratcliffe, P. J. (2004) Nat. Rev. Mol. Cell Biol. 5, 343–354. - PubMed
-
- Kaelin Jr., W. G. (2005) Annu. Rev. Biochem. 74, 115–128. - PubMed
-
- Taylor, M. S. (2001) Gene 275, 125–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

