Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo
- PMID: 16373503
- PMCID: PMC1324995
- DOI: 10.1073/pnas.0509054103
Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo
Abstract
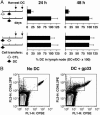
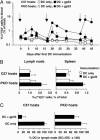
The lifespan and survival of dendritic cells (DC) in vivo are potentially critical to the expansion of T cell immune responses. We have previously reported that DC loaded with specific antigen are rapidly eliminated by cytotoxic T lymphocytes (CTL) in vivo, but the site, mechanism, and consequences of DC elimination were not defined. In this article we show that DC elimination in vivo occurs in a perforin-dependent manner and does not require IFN-gamma or the presence of CD4(+)CD25(+) regulatory T cells. Most importantly, failure to eliminate DC had profound consequences on the CTL immune response. Perforin-deficient mice showed a progressive increase in the numbers of antigen-specific CD8(+) T cells after repeated immunizations with DC. In contrast, in control mice the number of antigen-specific CD8(+) T cells did not notably increase with repeated immunizations. Lastly, we also show that CTL-mediated elimination of DC occurs in peripheral tissues but not in the lymph node. Our data suggest that CTL act as "gatekeepers" that control access of antigen-loaded DC into the lymph node, thereby preventing continued expansion of antigen-specific T cells.
Figures



Similar articles
-
Comparative evaluation of CD8+CTL responses following gene gun immunization targeting the skin with intracutaneous injection of antigen-transduced dendritic cells.Eur J Cell Biol. 2007 Dec;86(11-12):817-26. doi: 10.1016/j.ejcb.2006.07.002. Epub 2006 Aug 22. Eur J Cell Biol. 2007. PMID: 16928407
-
CD4 T cell help is required for primary CD8 T cell responses to vesicular antigen delivered to dendritic cells in vivo.Eur J Immunol. 2006 Jun;36(6):1386-97. doi: 10.1002/eji.200526193. Eur J Immunol. 2006. PMID: 16673447
-
Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes.Immunity. 2010 Feb 26;32(2):266-78. doi: 10.1016/j.immuni.2009.11.015. Epub 2010 Feb 4. Immunity. 2010. PMID: 20137985
-
[Role of CTL in liver injury of patients with HCV infection].Nihon Rinsho. 2004 Jul;62 Suppl 7(Pt 1):159-63. Nihon Rinsho. 2004. PMID: 15359785 Review. Japanese. No abstract available.
-
Perforin and lymphohistiocytic proliferative disorders.Br J Haematol. 2005 Mar;128(6):739-50. doi: 10.1111/j.1365-2141.2004.05305.x. Br J Haematol. 2005. PMID: 15755277 Review.
Cited by
-
Oxidant generation by single infected monocytes after short-term fluorescence labeling of a protozoan parasite.Infect Immun. 2007 Feb;75(2):1017-24. doi: 10.1128/IAI.00914-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118986 Free PMC article.
-
Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo.PLoS One. 2011;6(6):e20189. doi: 10.1371/journal.pone.0020189. Epub 2011 Jun 2. PLoS One. 2011. PMID: 21674051 Free PMC article.
-
Graded defects in cytotoxicity determine severity of hemophagocytic lymphohistiocytosis in humans and mice.Front Immunol. 2013 Dec 16;4:448. doi: 10.3389/fimmu.2013.00448. eCollection 2013. Front Immunol. 2013. PMID: 24379813 Free PMC article.
-
AIM2 regulates anti-tumor immunity and is a viable therapeutic target for melanoma.J Exp Med. 2021 Sep 6;218(9):e20200962. doi: 10.1084/jem.20200962. Epub 2021 Jul 29. J Exp Med. 2021. PMID: 34325468 Free PMC article.
-
Programmed cell death of dendritic cells in immune regulation.Immunol Rev. 2010 Jul;236:11-27. doi: 10.1111/j.1600-065X.2010.00916.x. Immunol Rev. 2010. PMID: 20636805 Free PMC article. Review.
References
-
- Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. - PubMed
-
- Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621–667. - PubMed
-
- Ruedl, C., Koebel, P., Bachmann, M., Hess, M. & Karjalainen, K. (2000) J. Immunol. 165, 4910–4916. - PubMed
-
- Kamath, A. T., Henri, S., Battye, F., Tough, D. F. & Shortman, K. (2002) Blood 100, 1734–1741. - PubMed
-
- Garg, S., Oran, A., Wajchman, J., Sasaki, S., Maris, C. H., Kapp, J. A. & Jacob, J. (2003) Nat. Immunol. 4, 907–912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

