Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes
- PMID: 16373506
- PMCID: PMC1324988
- DOI: 10.1073/pnas.0507407103
Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes
Abstract
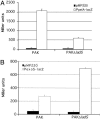
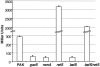
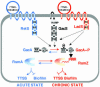
The opportunistic pathogen Pseudomonas aeruginosa is responsible for a wide range of acute and chronic infections. The transition to chronic infections is accompanied by physiological changes in the bacteria favoring formation of biofilm communities. Here we report the identification of LadS, a hybrid sensor kinase that controls the reciprocal expression of genes for type III secretion and biofilm-promoting polysaccharides. Domain organization of LadS and the range of LadS-controlled genes suggest that it counteracts the activities of another sensor kinase, RetS. These two pathways converge by controlling the transcription of a small regulatory RNA, RsmZ. This work identifies a previously undescribed signal transduction network in which the activities of signal-receiving sensor kinases LadS, RetS, and GacS regulate expression of virulence genes associated with acute or chronic infection by transcriptional and posttranscriptional mechanisms.
Figures


Similar articles
-
A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa.Dev Cell. 2004 Nov;7(5):745-54. doi: 10.1016/j.devcel.2004.08.020. Dev Cell. 2004. PMID: 15525535
-
Mutational analysis of RetS, an unusual sensor kinase-response regulator hybrid required for Pseudomonas aeruginosa virulence.Infect Immun. 2006 Aug;74(8):4462-73. doi: 10.1128/IAI.00575-06. Infect Immun. 2006. PMID: 16861632 Free PMC article.
-
Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS.Mol Microbiol. 2013 May;88(4):784-97. doi: 10.1111/mmi.12223. Epub 2013 Apr 21. Mol Microbiol. 2013. PMID: 23560772
-
The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms.Biomed Res Int. 2015;2015:759348. doi: 10.1155/2015/759348. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866808 Free PMC article. Review.
-
Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa.FEMS Microbiol Rev. 2009 Mar;33(2):279-94. doi: 10.1111/j.1574-6976.2008.00135.x. FEMS Microbiol Rev. 2009. PMID: 19243444 Review.
Cited by
-
A chemical biology approach to interrogate quorum-sensing regulated behaviors at the molecular and cellular level.Chem Biol. 2013 Jul 25;20(7):903-11. doi: 10.1016/j.chembiol.2013.05.009. Chem Biol. 2013. PMID: 23890008 Free PMC article.
-
Unique features of a Pseudomonas aeruginosa α2-macroglobulin homolog.mBio. 2013 Aug 6;4(4):e00309-13. doi: 10.1128/mBio.00309-13. mBio. 2013. PMID: 23919994 Free PMC article.
-
Polyamines and linear DNA mediate bacterial threat assessment of bacteriophage infection.Proc Natl Acad Sci U S A. 2023 Feb 28;120(9):e2216430120. doi: 10.1073/pnas.2216430120. Epub 2023 Feb 21. Proc Natl Acad Sci U S A. 2023. PMID: 36802441 Free PMC article.
-
Identification of small RNAs in Francisella tularensis.BMC Genomics. 2010 Nov 10;11:625. doi: 10.1186/1471-2164-11-625. BMC Genomics. 2010. PMID: 21067590 Free PMC article.
-
Profil bactériologique et résistance aux antibiotiques des bactéries isolées dans un service de réanimation des brûlés durant sept ans.Ann Burns Fire Disasters. 2019 Sep 30;32(3):197-202. Ann Burns Fire Disasters. 2019. PMID: 32313533 Free PMC article. French.
References
-
- Nickel, J. C., Downey, J. A. & Costerton, J. W. (1989) Urology 34, 284–291. - PubMed
-
- Fletcher, E. L., Weissman, B. A., Efron, N., Fleiszig, S. M., Curcio, A. J. & Brennan, N. A. (1993) Curr. Eye Res. 12, 1067–1071. - PubMed
-
- O'Toole, G., Kaplan, H. B. & Kolter, R. (2000) Annu. Rev. Microbiol. 54, 49–79. - PubMed
-
- Mah, T. F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S. & O'Toole, G. A. (2003) Nature 426, 306–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

