Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction
- PMID: 16374515
- PMCID: PMC1319219
- DOI: 10.1172/JCI25423
Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction
Abstract
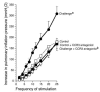
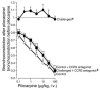
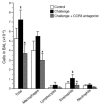
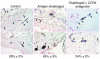
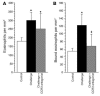
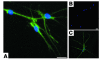
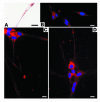
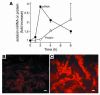
Eosinophils cluster around airway nerves in patients with fatal asthma and in antigen-challenged animals. Activated eosinophils release major basic protein, which blocks inhibitory M2 muscarinic receptors (M2Rs) on nerves, increasing acetylcholine release and potentiating vagally mediated bronchoconstriction. We tested whether GW701897B, an antagonist of CCR3 (the receptor for eotaxin as well as a group of eosinophil active chemokines), affected vagal reactivity and M2R function in ovalbumin-challenged guinea pigs. Sensitized animals were treated with the CCR3 antagonist before inhaling ovalbumin. Antigen-challenged animals were hyperresponsive to vagal stimulation, but those that received the CCR3 antagonist were not. M2R function was lost in antigen-challenged animals, but not in those that received the CCR3 antagonist. Although the CCR3 antagonist did not decrease the number of eosinophils in lung tissues as assessed histologically, CCR3 antagonist prevented antigen-induced clustering of eosinophils along the nerves. Immunostaining revealed eotaxin in airway nerves and in cultured airway parasympathetic neurons from both guinea pigs and humans. Both IL-4 and IL-13 increased expression of eotaxin in cultured airway parasympathetic neurons as well as in human neuroblastoma cells. Thus, signaling via CCR3 mediates eosinophil recruitment to airway nerves and may be a prerequisite to blockade of inhibitory M2Rs by eosinophil major basic protein.
Figures





References
-
- Minette P, Barnes PJ. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea-pig airways. J. Appl. Physiol. 1988;64:2532–2537. - PubMed
-
- Minette PJ, Lammers JWJ, Dixon CMS, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not asthmatic subjects. J. Appl. Physiol. 1989;67:2461–2465. - PubMed
-
- Costello RW, et al. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am. J. Physiol. 1997;273:L93–L103. - PubMed

