Abnormal expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in hippocampus, frontal cortex, and substantia nigra of Ts65Dn mouse: a model of Down syndrome
- PMID: 16374808
- PMCID: PMC2929960
- DOI: 10.1002/cne.20844
Abnormal expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in hippocampus, frontal cortex, and substantia nigra of Ts65Dn mouse: a model of Down syndrome
Abstract
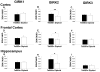
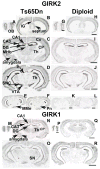
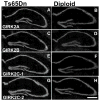
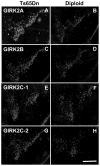
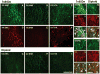
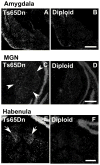
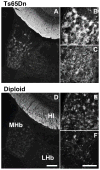
Ts65Dn, a mouse model of Down syndrome (DS), demonstrates abnormal hippocampal synaptic plasticity and behavioral abnormalities related to spatial learning and memory. The molecular mechanisms leading to these impairments have not been identified. In this study, we focused on the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) gene that is highly expressed in the hippocampus region. We studied the expression pattern of GIRK subunits in Ts65Dn and found that GIRK2 was overexpressed in all analyzed Ts65Dn brain regions. Interestingly, elevated levels of GIRK2 protein in the Ts65Dn hippocampus and frontal cortex correlated with elevated levels of GIRK1 protein. This suggests that heteromeric GIRK1-GIRK2 channels are overexpressed in Ts65Dn hippocampus and frontal cortex, which could impair excitatory input and modulate spike frequency and synaptic kinetics in the affected regions. All GIRK2 splicing isoforms examined were expressed at higher levels in the Ts65Dn in comparison to the diploid hippocampus. The pattern of GIRK2 expression in the Ts65Dn mouse brain revealed by in situ hybridization and immunohistochemistry was similar to that previously reported in the rodent brain. However, in the Ts65Dn mouse a strong immunofluorescent staining of GIRK2 was detected in the lacunosum molecular layer of the CA3 area of the hippocampus. In addition, tyrosine hydroxylase containing dopaminergic neurons that coexpress GIRK2 were more numerous in the substantia nigra compacta and ventral tegmental area in the Ts65Dn compared to diploid controls. In summary, the regional localization and the increased brain levels coupled with known function of the GIRK channel may suggest an important contribution of GIRK2 containing channels to Ts65Dn and thus to DS neurophysiological phenotypes.
Copyright 2005 Wiley-Liss, Inc.
Figures





References
-
- Adeniji-Adele A, Harashima C, Best T, Stoffel M, Galdzicki Z, Siarey RJ. Long-term potentiation and depression in the GIRK2−/− mouse. 34rd Annual Meeting of Society for Neuroscience; 2004; 2004. p. 56.16.
-
- Antonarakis SE. Parental origin of the extra chromosome in trisomy 21 as indicated by analysis of DNA polymorphisms. Down Syndrome Collaborative Group. N Engl J Med. 1991;324:872–876. - PubMed
-
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–298. - PubMed
-
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

