Pathogen inactivation techniques
- PMID: 16377551
- PMCID: PMC7106341
- DOI: 10.1016/j.beha.2005.04.001
Pathogen inactivation techniques
Abstract
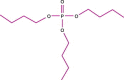
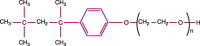
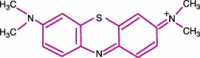
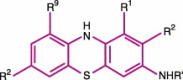
The desire to rid the blood supply of pathogens of all types has led to the development of many technologies aimed at the same goal--eradication of the pathogen(s) without harming the blood cells or generating toxic chemical agents. This is a very ambitious goal, and one that has yet to be achieved. One approach is to shun the 'one size fits all' concept and to target pathogen-reduction agents at the Individual component types. This permits the development of technologies that might be compatible with, for example, plasma products but that would be cytocidal and thus incompatible with platelet concentrates or red blood cell units. The technologies to be discussed include solvent detergent and methylene blue treatments--designed to inactivate plasma components and derivatives; psoralens (S-59--amotosalen) designed to pathogen-reduce units of platelets; and two products aimed at red blood cells, S-303 (a Frale--frangible anchor-linker effector compound) and Inactine (a binary ethyleneimine). A final pathogen-reduction material that might actually allow one material to inactivate all three blood components--riboflavin (vitamin B2)--is also under development. The sites of action of the amotosalen (S-59), the S-303 Frale, Inactine, and riboflavin are all localized in the nucleic acid part of the pathogen. Solvent detergent materials act by dissolving the plasma envelope, thus compromising the integrity of the pathogen membrane and rendering it non-infectious. By disrupting the pathogen's ability to replicate or survive, its infectivity is removed. The degree to which bacteria and viruses are affected by a particular pathogen-reducing technology relates to its Gram-positive or Gram-negative status, to the sporulation characteristics for bacteria, and the presence of lipid or protein envelopes for viruses. Concerns related to photoproducts and other breakdown products of these technologies remain, and the toxicology of pathogen-reduction treatments is a major ongoing area of investigation. Clearly, regulatory agencies have a major role to play in the evaluation of these new technologies. This chapter will cover the several types of pathogen-reduction systems, mechanisms of action, the inactivation efficacy for specific types of pathogens, toxicology of the various systems and the published research and clinical trial data supporting their potential usefulness. Due to the nature of the field, pathogen reduction is a work in progress and this review should be considered as a snapshot in time rather than a clear picture of what the future will bring.
Figures

References
-
- Teitel J.M. Viral safety of haemophilia treatment products. Annals Medicine. 2000;32(7):785. - PubMed
-
- Epstein J.S., Vostal J.G. FDA approach to evaluation of pathogen reduction technology. Transfusion. 2003;43:1347. - PubMed
-
- Katz L. Blood bags for diversion of the initial collection: a statement of the American Association of Blood Banks before the Blood Products Advisory Committee; March 15, 2001.
-
- Rossi E.C., Simon T.L. Transfusion in the new millennium. In: Simon T.L., editor. In: Rossi's Principles of Transfusion Medicine. third edn. Lippincott Williams and Wilkins; Philadelphia, PA: 2002.
-
- Technical Manual. 14th edn. American Association of Blood Banks; Bethesda, MD: 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

