Posaconazole as salvage therapy for zygomycosis
- PMID: 16377677
- PMCID: PMC1346806
- DOI: 10.1128/AAC.50.1.126-133.2006
Posaconazole as salvage therapy for zygomycosis
Abstract
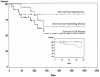
Zygomycosis, an infection that is associated with significant morbidity and mortality, is becoming common in immunocompromised patients. Posaconazole is a new extended-spectrum azole antifungal that has demonstrated in vitro and in vivo activity against zygomycetes. This report provides the results from the first 24 patients with active zygomycosis who were enrolled in two open-label, nonrandomized, multicentered compassionate trials that evaluated oral posaconazole as salvage therapy for invasive fungal infections. Posaconazole was usually given as an oral suspension of 200 mg four times a day or 400 mg twice a day. Eleven (46%) of the infections were rhinocerebral. Duration of posaconazole therapy ranged from 8 to 1,004 days (mean, 292 days; median, 182 days). Rates of successful treatment (complete cure and partial response) were 79% in 19 subjects with zygomycosis refractory to standard therapy and 80% in 5 subjects with intolerance to standard therapy. Overall, 19 of 24 subjects (79%) survived infection. Survival was also associated with surgical resection of affected tissue and stabilization or improvement of the subjects' underlying illnesses. Failures either had worsening of underlying illnesses or requested all therapy withdrawn; none of the failures received more than 31 days of posaconazole. Posaconazole oral solution was well tolerated and was discontinued in only one subject due to a drug rash. Posaconazole appears promising as an oral therapy for zygomycosis in patients who receive required surgery and control their underlying illness.
Figures
References
-
- Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. - PubMed
-
- Barrett, J. P., K. A. Vardulaki, C. Conlon, J. Cooke, P. Daza-Ramirez, E. G. Evans, P. M. Hawkey, R. Herbrecht, D. I. Marks, J. M. Moraleda, G. R. Park, S. J. Senn, and C. Viscoli. 2003. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clin. Ther. 25:1295-1320. - PubMed
-
- Chakrabarti, A., A. Das, A. Sharma, N. Panda, S. Das, K. L. Gupta, and V. Sakhuja. 2001. Ten years' experience in zygomycosis at a tertiary care centre in India. J. Infect. 42:261-266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

