Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins
- PMID: 16377746
- PMCID: PMC1326053
- DOI: 10.1104/pp.105.070219
Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins
Abstract
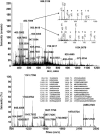
Cell wall proteins (CWPs) play important roles in various processes, including cell elongation. However, relatively little is known about the composition of CWPs in growing regions. We are using a proteomics approach to gain a comprehensive understanding of the identity of CWPs in the maize (Zea mays) primary root elongation zone. As the first step, we examined the effectiveness of a vacuum infiltration-centrifugation technique for extracting water-soluble and loosely ionically bound (fraction 1) CWPs from the root elongation zone. The purity of the CWP extract was evaluated by comparing with total soluble proteins extracted from homogenized tissue. Several lines of evidence indicated that the vacuum infiltration-centrifugation technique effectively enriched for CWPs. Protein identification revealed that 84% of the CWPs were different from the total soluble proteins. About 40% of the fraction 1 CWPs had traditional signal peptides and 33% were predicted to be nonclassical secretory proteins, whereas only 3% and 11%, respectively, of the total soluble proteins were in these categories. Many of the CWPs have previously been shown to be involved in cell wall metabolism and cell elongation. In addition, maize has type II cell walls, and several of the CWPs identified in this study have not been identified in previous cell wall proteomics studies that have focused only on type I walls. These proteins include endo-1,3;1,4-beta-D-glucanase and alpha-L-arabinofuranosidase, which act on the major polysaccharides only or mainly present in type II cell walls.
Figures



References
-
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 - PubMed
-
- Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S (2004) Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel 17: 349–356 - PubMed
-
- Blee KA, Wheatley ER, Bonham VA, Mitchell GP, Robertson D, Slabas AR, Burrell MM, Wojtaszek P, Bolwell GP (2001) Proteomic analysis reveals a novel set of cell wall proteins in a transformed tobacco cell culture that synthesises secondary walls as determined by biochemical and morphological parameters. Planta 212: 404–415 - PubMed
-
- Borderies G, Jamet E, Lafitte C, Rossignol M, Jauneau A, Boudart G, Monsarrat B, Esquerre-Tugaye MT, Boudet A, Pont-Lezica R (2003) Proteomics of loosely bound cell wall proteins of Arabidopsis thaliana cell suspension cultures: a critical analysis. Electrophoresis 24: 3421–3432 - PubMed
-
- Boudart G, Jamet E, Rossignol M, Lafitte C, Borderies G, Jauneau A, Esquerre-Tugaye MT, Pont-Lezica R (2005) Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: identification by mass spectrometry and bioinformatics. Proteomics 5: 212–221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

