A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta-hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth
- PMID: 16377748
- PMCID: PMC1326029
- DOI: 10.1104/pp.105.069690
A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta-hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth
Erratum in
- Plant Physiol. 2006 Aug;141(4):1708
- Plant Physiol. 2007 Apr;143(4):1982
Abstract
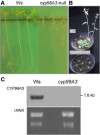
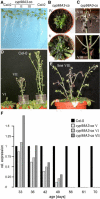
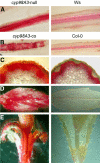
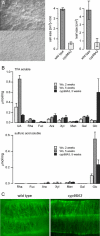
Cytochromes P450 monooxygenases from the CYP98 family catalyze the meta-hydroxylation step in the phenylpropanoid biosynthetic pathway. The ref8 Arabidopsis (Arabidopsis thaliana) mutant, with a point mutation in the CYP98A3 gene, was previously described to show developmental defects, changes in lignin composition, and lack of soluble sinapoyl esters. We isolated a T-DNA insertion mutant in CYP98A3 and show that this mutation leads to a more drastic inhibition of plant development and inhibition of cell growth. Similar to the ref8 mutant, the insertion mutant has reduced lignin content, with stem lignin essentially made of p-hydroxyphenyl units and trace amounts of guaiacyl and syringyl units. However, its roots display an ectopic lignification and a substantial proportion of guaiacyl and syringyl units, suggesting the occurrence of an alternative CYP98A3-independent meta-hydroxylation mechanism active mainly in the roots. Relative to the control, mutant plantlets produce very low amounts of sinapoyl esters, but accumulate flavonol glycosides. Reduced cell growth seems correlated with alterations in the abundance of cell wall polysaccharides, in particular decrease in crystalline cellulose, and profound modifications in gene expression and homeostasis reminiscent of a stress response. CYP98A3 thus constitutes a critical bottleneck in the phenylpropanoid pathway and in the synthesis of compounds controlling plant development. CYP98A3 cosuppressed lines show a gradation of developmental defects and changes in lignin content (40% reduction) and structure (prominent frequency of p-hydroxyphenyl units), but content in foliar sinapoyl esters is similar to the control. The purple coloration of their leaves is correlated to the accumulation of sinapoylated anthocyanins.
Figures




References
-
- Adler E, Bjorkquist KJ, Haggroth S (1948) Ûber die Ursache der Farbreaktionen des Holzes. Acta Chem Scand 2: 93–94
-
- Albersheim P, Nevins DJ, English PD, Karr A (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr Res 5: 340–345
-
- Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215: 150–171 - PubMed
-
- Bloor SJ, Abrahams S (2002) The structure of the major anthocyanin in Arabidopsis thaliana. Phytochemistry 59: 343–346 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

