The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception
- PMID: 16377758
- PMCID: PMC1356552
- DOI: 10.1105/tpc.105.036574
The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception
Abstract
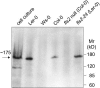
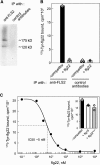
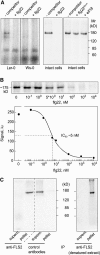
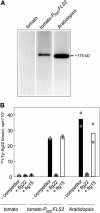
Flagellin, the main building block of the bacterial flagellum, acts as a pathogen-associated molecular pattern triggering the innate immune response in animals and plants. In Arabidopsis thaliana, the Leu-rich repeat transmembrane receptor kinase FLAGELLIN SENSITIVE2 (FLS2) is essential for flagellin perception. Here, we demonstrate the specific interaction of the elicitor-active epitope flg22 with the FLS2 protein by chemical cross-linking and immunoprecipitation. The functionality of this receptor was further tested by heterologous expression of the Arabidopsis FLS2 gene in tomato (Lycopersicon esculentum) cells. The perception of flg22 in tomato differs characteristically from that in Arabidopsis. Expression of Arabidopsis FLS2 conferred an additional flg22-perception system on the cells of tomato, which showed all of the properties characteristic of the perception of this elicitor in Arabidopsis. In summary, these results show that FLS2 constitutes the pattern-recognition receptor that determines the specificity of flagellin perception.
Figures
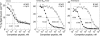
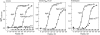

References
-
- Bauer, Z., Gómez-Gómez, L., Boller, T., and Felix, G. (2001). Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J. Biol. Chem. 276 45669–45676. - PubMed
-
- Che, F.S., Nakajima, Y., Tanaka, N., Iwano, M., Yoshida, T., Takayama, S., Kadota, I., and Isogai, A. (2000). Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 275 32347–32356. - PubMed
-
- Evan, G.I., and Hancock, D.C. (1985). Studies on the interaction of the human c-myc protein with cell nuclei: p62c-myc as a member of a discrete subset of nuclear proteins. Cell 43 253–261. - PubMed
-
- Felix, G., Duran, J.D., Volko, S., and Boller, T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18 265–276. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

