Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function
- PMID: 16378973
- PMCID: PMC1346846
- DOI: 10.1128/JVI.80.2.697-709.2006
Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function
Abstract
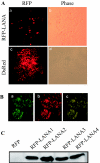
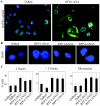
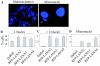
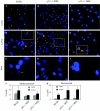
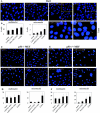
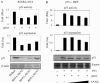
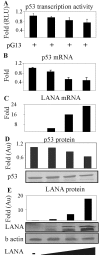
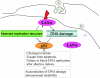
Kaposi's sarcoma-associated herpesvirus (KSHV) is predominantly associated with three human malignancies, KS, primary effusion lymphoma, and multicentric Castleman's disease. These disorders are linked to genomic instability, known to be a crucial component of the oncogenic process. Latency-associated nuclear antigen (LANA), encoded by open reading frame 73 of the KSHV genome, is a latent protein consistently expressed in all KSHV-associated diseases. LANA is important in viral genome maintenance and is associated with cellular and viral proteins to regulate viral and cellular gene expression. LANA interacts with the tumor suppressor genes p53 and pRb, indicating that LANA may target these proteins and promote oncogenesis. In this study, we generated cell lines which stably expressed LANA to observe the effects of LANA expression on cell phenotype. LANA expression in these stable cell lines showed a dramatic increase in chromosomal instability, indicated by the presence of increased multinucleation, micronuclei, and aberrant centrosomes. In addition, these stable cell lines demonstrated an increased proliferation rate and as well as increased entry into S phase in both stable and transiently transfected LANA-expressing cells. Additionally, p53 transcription and its transactivation activity were suppressed by LANA expression in a dose-dependent manner. LANA may therefore promote chromosomal instability by suppressing the functional activities of p53, thereby facilitating KSHV-mediated pathogenesis and cancer.
Figures


References
-
- An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. - PubMed
-
- Attardi, L. D. 2005. The role of p53-mediated apoptosis as a crucial anti-tumor response to genomic instability: lessons from mouse models. Mutat. Res. Fund. Mol. Mech. Mutagen. 569:145-157. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
-
- Blagosklonny, M. V., P. Giannakakou, L. Y. Romanova, K. M. Ryan, K. H. Vousden, and T. Fojo. 2001. Inhibition of HIF-1- and wild-type p53-stimulated transcription by codon Arg175 p53 mutants with selective loss of functions. Carcinogenesis 22:861-867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

