Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome
- PMID: 16379002
- PMCID: PMC1346861
- DOI: 10.1128/JVI.80.2.1015-1024.2006
Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome
Abstract
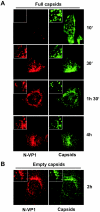
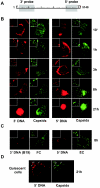
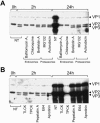
Minute virus of mice (MVM) enters the host cell via receptor-mediated endocytosis. Although endosomal processing is required, its role remains uncertain. In particular, the effect of low endosomal pH on capsid configuration and nuclear delivery of the viral genome is unclear. We have followed the progression and structural transitions of DNA full-virus capsids (FC) and empty capsids (EC) containing the VP1 and VP2 structural proteins and of VP2-only virus-like particles (VLP) during the endosomal trafficking. Three capsid rearrangements were detected in FC: externalization of the VP1 N-terminal sequence (N-VP1), cleavage of the exposed VP2 N-terminal sequence (N-VP2), and uncoating of the full-length genome. All three capsid modifications occurred simultaneously, starting as early as 30 min after internalization, and all of them were blocked by raising the endosomal pH. In particles lacking viral single-stranded DNA (EC and VLP), the N-VP2 was not exposed and thus it was not cleaved. However, the EC did externalize N-VP1 with kinetics similar to those of FC. The bulk of all the incoming particles (FC, EC, and VLP) accumulated in lysosomes without signs of lysosomal membrane destabilization. Inside lysosomes, capsid degradation was not detected, although the uncoated DNA of FC was slowly degraded. Interestingly, at any time postinfection, the amount of structural proteins of the incoming virions accumulating in the nuclear fraction was negligible. These results indicate that during the early endosomal trafficking, the MVM particles are structurally modified by low-pH-dependent mechanisms. Regardless of the structural transitions and protein composition, the majority of the entering viral particles and genomes end in lysosomes, limiting the efficiency of MVM nuclear translocation.
Figures





References
-
- Basak, S., and H. Turner. 1992. Infectious entry pathway for canine parvovirus. Virology 186:368-376. - PubMed
-
- Canaan, S., Z. Zadori, F. Ghomashchi, J. Bollinger, M. Sadilek, M. E. Moreau, P. Tijssen, and M. H. Gelb. 2004. Interfacial enzymology of parvovirus phospholipases A2. J. Biol. Chem. 279:14502-14508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

