Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells
- PMID: 16380438
- PMCID: PMC2063537
- DOI: 10.1083/jcb.200508044
Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells
Erratum in
- J Cell Biol. 2007 Jan 1;176(1):125
Abstract
The growth and repair of skeletal muscle after birth depends on satellite cells that are characterized by the expression of Pax7. We show that Pax3, the paralogue of Pax7, is also present in both quiescent and activated satellite cells in many skeletal muscles. Dominant-negative forms of both Pax3 and -7 repress MyoD, but do not interfere with the expression of the other myogenic determination factor, Myf5, which, together with Pax3/7, regulates the myogenic differentiation of these cells. In Pax7 mutants, satellite cells are progressively lost in both Pax3-expressing and -nonexpressing muscles. We show that this is caused by satellite cell death, with effects on the cell cycle. Manipulation of the dominant-negative forms of these factors in satellite cell cultures demonstrates that Pax3 cannot replace the antiapoptotic function of Pax7. These findings underline the importance of cell survival in controlling the stem cell populations of adult tissues and demonstrate a role for upstream factors in this context.
Figures

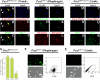




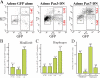
References
-
- Abrams, J.M., and M.A. White. 2004. Coordination of cell death and the cell cycle: linking proliferation to death through private and communal couplers. Curr. Opin. Cell Biol. 16:634–638. - PubMed
-
- Auerbach, R. 1954. Analysis of the developmental effects of a lethal mutation in the house mouse. J. Exp. Zool. 127:305–329.
-
- Ben-Yair, R., and C. Kalcheim. 2005. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development. 132:689–701. - PubMed
-
- Bischoff, R., and C. Heintz. 1994. Enhancement of skeletal muscle regeneration. Dev. Dyn. 201:41–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

