Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane
- PMID: 16381811
- PMCID: PMC1382309
- DOI: 10.1091/mbc.e05-07-0643
Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane
Abstract
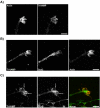
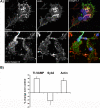
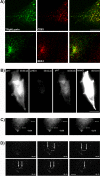
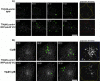
Tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP)-mediated fusion of intracellular vesicles with the plasma membrane is crucial for neurite outgrowth, a pathway not requiring synaptobrevin-dependent exocytosis. Yet, it is not known how the TI-VAMP membrane trafficking pathway is regulated or how it is coordinated with cytoskeletal dynamics within the growth cone that guide neurite outgrowth. Here, we demonstrate that TI-VAMP, but not synaptobrevin 2, concentrates in the peripheral, F-actin-rich region of the growth cones of hippocampal neurons in primary culture. Its accumulation correlates with and depends upon the presence of F-actin. Moreover, acute stimulation of actin remodeling by homophilic activation of the adhesion molecule L1 induces a site-directed, actin-dependent recruitment of the TI-VAMP compartment. Expression of a dominant-positive mutant of Cdc42, a key regulator of cell polarity, stimulates formation of F-actin- and TI-VAMP-rich filopodia outside the growth cone. Furthermore, we report that Cdc42 activates exocytosis of pHLuorin tagged TI-VAMP in an actin-dependent manner. Collectively, our data suggest that Cdc42 and regulated assembly of the F-actin network control the accumulation and exocytosis of TI-VAMP-containing membrane vesicles in growth cones to coordinate membrane trafficking and actin remodeling during neurite outgrowth.
Figures




References
-
- Bradke, F., and Dotti, C. G. (1999). The role of local actin instability in axon formation. Science 283, 1931–1934. - PubMed
-
- Brown, M. D., Cornejo, B. J., Kuhn, T. B., and Bamburg, J. R. (2000). Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol. 43, 352–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

