Regulation of sexual dimorphism: mutational and chemogenetic analysis of the doublesex DM domain
- PMID: 16382145
- PMCID: PMC1346899
- DOI: 10.1128/MCB.26.2.535-547.2006
Regulation of sexual dimorphism: mutational and chemogenetic analysis of the doublesex DM domain
Abstract
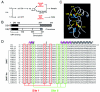
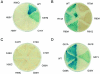
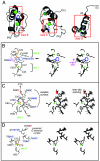
Doublesex (dsx) is a transcription factor in Drosophila that regulates somatic sexual differentiation. Male- and female-specific splicing isoforms of DSX share a novel DNA-binding domain, designated the DM motif. Broadly conserved among metazoan sex-determining factors, the DM domain contains a nonclassical zinc module and binds in the DNA minor groove. Here, we characterize the DM motif by site-directed and random mutagenesis using a yeast one-hybrid (Y1H) system and extend this analysis by chemogenetic complementation in vitro. The Y1H system is based on a sex-specific Drosophila enhancer element and validated through studies of intersexual dsx mutations. We demonstrate that the eight motif-specific histidines and cysteines engaged in zinc coordination are each critical and cannot be interchanged; folding also requires conserved aliphatic side chains in the hydrophobic core. Mutations that impair DNA binding tend to occur at conserved positions, whereas neutral substitutions occur at nonconserved sites. Evidence for a specific salt bridge between a conserved lysine and the DNA backbone is obtained through the synthesis of nonstandard protein and DNA analogs. Together, these results provide molecular links between the structure of the DM domain and its function in the regulation of sexual dimorphism.
Figures







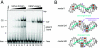
Similar articles
-
Doublesex and the regulation of sexual dimorphism in Drosophila melanogaster: structure, function, and mutagenesis of a female-specific domain.J Biol Chem. 2008 Mar 14;283(11):7280-92. doi: 10.1074/jbc.M708742200. Epub 2008 Jan 9. J Biol Chem. 2008. PMID: 18184648
-
Dimerization of doublesex is mediated by a cryptic ubiquitin-associated domain fold: implications for sex-specific gene regulation.J Biol Chem. 2005 Sep 23;280(38):32989-96. doi: 10.1074/jbc.M507990200. Epub 2005 Jul 27. J Biol Chem. 2005. PMID: 16049008
-
Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers.Genes Dev. 2000 Jul 15;14(14):1750-64. Genes Dev. 2000. PMID: 10898790 Free PMC article.
-
Double nexus--Doublesex is the connecting element in sex determination.Brief Funct Genomics. 2015 Nov;14(6):396-406. doi: 10.1093/bfgp/elv005. Epub 2015 Mar 22. Brief Funct Genomics. 2015. PMID: 25797692 Free PMC article. Review.
-
The roles of fruitless and doublesex in the control of male courtship.Int Rev Neurobiol. 2011;99:87-105. doi: 10.1016/B978-0-12-387003-2.00004-5. Int Rev Neurobiol. 2011. PMID: 21906537 Review.
Cited by
-
Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development.Development. 2011 Jul;138(13):2761-71. doi: 10.1242/dev.065227. Development. 2011. PMID: 21652649 Free PMC article.
-
Batesian mimicry has evolved with deleterious effects of the pleiotropic gene doublesex.Sci Rep. 2020 Dec 7;10(1):21333. doi: 10.1038/s41598-020-78055-1. Sci Rep. 2020. PMID: 33288816 Free PMC article.
-
A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1(-/-) testes: a novel model of DMRT1-deficient germ cells.Biol Reprod. 2013 Feb 1;88(2):51. doi: 10.1095/biolreprod.112.103135. Print 2013 Feb. Biol Reprod. 2013. PMID: 23255335 Free PMC article.
-
The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle.EMBO Rep. 2013 Jun;14(6):561-7. doi: 10.1038/embor.2013.50. Epub 2013 Apr 23. EMBO Rep. 2013. PMID: 23609854 Free PMC article.
-
Crystallographic analysis of a sex-specific enhancer element: sequence-dependent DNA structure, hydration, and dynamics.J Mol Biol. 2009 Jan 16;385(2):469-90. doi: 10.1016/j.jmb.2008.10.041. Epub 2008 Oct 22. J Mol Biol. 2009. PMID: 18992257 Free PMC article.
References
-
- Ahmad, S. M., and B. S. Baker. 2002. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109:651-661. - PubMed
-
- An, W., and P. C. Wensink. 1995. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 9:256-266. - PubMed
-
- Bayrer, J. R., W. Zhang, and M. A. Weiss. 2005. Dimerization of doublesex is mediated by a cryptic UBA-domain fold. Implications for sex-specific gene regulation. J. Biol. Chem. 280:32989-32996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
