Acid destabilization of the solution conformation of Bcl-xL does not drive its pH-dependent insertion into membranes
- PMID: 16385002
- PMCID: PMC1752203
- DOI: 10.1110/ps.051807706
Acid destabilization of the solution conformation of Bcl-xL does not drive its pH-dependent insertion into membranes
Abstract
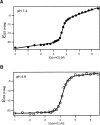
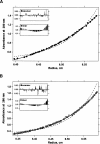
Regulation of programmed cell death by Bcl-xL is dependent on both its solution and integral membrane conformations. A conformational change from solution to membrane is also important in this regulation. This conformational change shows a pH-dependence similar to the translocation domain of diphtheria toxin, where an acid-induced molten globule conformation in the absence of lipid vesicles mediates the change from solution to membrane conformations. By contrast, Bcl-xL deltaTM in the absence of lipid vesicles exhibits no gross conformational changes upon acidification as observed by near- and far-UV circular dichroism spectropolarimetry. Additionally, no significant local conformational changes upon acidification were observed by heteronuclear NMR spectroscopy of Bcl-xL deltaTM. Under conditions that favor the solution conformation (pH 7.4), the free energy of folding for Bcl-xL deltaTM (deltaG(o)) was determined to be 15.8 kcal x mol(-1). Surprisingly, under conditions that favor a membrane conformation (pH 4.9), deltaG(o) was 14.6 kcal x mol(-1). These results differ from those obtained with many other membrane-insertable proteins where acid-induced destabilization is important. Therefore, other contributions must be necessary to destabilize the solution conformation Bcl-xL and favor the membrane conformation at pH 4.9. Such contributions might include the presence of a negatively charged membrane or an electrostatic potential across the membrane. Thus, for proteins that adopt both solution and membrane conformations, an obligatory molten globule intermediate may not be necessary. The absence of a molten globule intermediate might have evolved to protect Bcl-xL from intracellular proteases as it undergoes this conformational change essential for its activity.
Figures




Similar articles
-
Evidence that membrane insertion of the cytosolic domain of Bcl-xL is governed by an electrostatic mechanism.J Mol Biol. 2006 Jun 16;359(4):1045-58. doi: 10.1016/j.jmb.2006.03.052. Epub 2006 Apr 6. J Mol Biol. 2006. PMID: 16650855 Free PMC article.
-
Comparison of membrane insertion pathways of the apoptotic regulator Bcl-xL and the diphtheria toxin translocation domain.Biochemistry. 2013 Nov 12;52(45):7901-9. doi: 10.1021/bi400926k. Epub 2013 Nov 1. Biochemistry. 2013. PMID: 24134052 Free PMC article.
-
Lipid-modulation of membrane insertion and refolding of the apoptotic inhibitor Bcl-xL.Biochim Biophys Acta Proteins Proteom. 2019 Jul-Aug;1867(7-8):691-700. doi: 10.1016/j.bbapap.2019.04.006. Epub 2019 Apr 18. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31004798 Free PMC article.
-
Conformational Switching in Bcl-xL: Enabling Non-Canonic Inhibition of Apoptosis Involves Multiple Intermediates and Lipid Interactions.Cells. 2020 Feb 26;9(3):539. doi: 10.3390/cells9030539. Cells. 2020. PMID: 32111007 Free PMC article.
-
Molten globule-state protein structure: Perspectives from food processing applications.Food Res Int. 2024 Dec;198:115318. doi: 10.1016/j.foodres.2024.115318. Epub 2024 Nov 12. Food Res Int. 2024. PMID: 39643361 Review.
Cited by
-
The N-terminal domain of Bcl-xL reversibly binds membranes in a pH-dependent manner.Biochemistry. 2006 Dec 5;45(48):14533-42. doi: 10.1021/bi0616652. Biochemistry. 2006. PMID: 17128992 Free PMC article.
-
Cathepsin S Cleaves BAX as a Novel and Therapeutically Important Regulatory Mechanism for Apoptosis.Pharmaceutics. 2021 Mar 5;13(3):339. doi: 10.3390/pharmaceutics13030339. Pharmaceutics. 2021. PMID: 33807987 Free PMC article.
-
Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1.J Biol Chem. 2008 Sep 19;283(38):26274-82. doi: 10.1074/jbc.M804723200. Epub 2008 Jul 18. J Biol Chem. 2008. PMID: 18641390 Free PMC article.
-
The variable domain from dynamin-related protein 1 promotes liquid-liquid phase separation that enhances its interaction with cardiolipin-containing membranes.Protein Sci. 2023 Nov;32(11):e4787. doi: 10.1002/pro.4787. Protein Sci. 2023. PMID: 37743569 Free PMC article.
-
Mapping the interaction of pro-apoptotic tBID with pro-survival BCL-XL.Biochemistry. 2009 Sep 15;48(36):8704-11. doi: 10.1021/bi901171n. Biochemistry. 2009. PMID: 19670908 Free PMC article.
References
-
- Ackers, G.K. 1998. Deciphering the molecular code of hemoglobin allostery. Adv. Protein Chem. 51: 185–253. - PubMed
-
- Adams, J.M. and Cory, S. 1998. The Bcl-2 protein family: Arbiters of cell survival. Science 281: 1322–1326. - PubMed
-
- Amezcua, C.A., Harper, S.M., Rutter, J., and Gardner, K.H. 2002. Structure and interactions of PAS kinase N-terminal PAS domain: Model for intramolecular kinase regulation. Structure (Camb.) 10: 1349–1361. - PubMed
-
- Antonsson, B., Montessuit, S., Sanchez, B., and Martinou, J.C. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615–11623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

