Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition
- PMID: 16385005
- PMCID: PMC2242455
- DOI: 10.1110/ps.051891106
Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition
Abstract
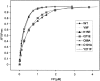
Glutathione S-transferase of the malarial parasite Plasmodium falciparum (PfGST) represents a novel class of GST isoenzymes. Since the architecture of the PfGST substrate binding site differs significantly from its human counterparts and there is only this one isoenzyme present in the parasite, PfGST is considered a highly attractive target for antimalarial drug development. Here we report the mechanistic, kinetic, and structural characterization of PfGST as well as its interaction with different ligands. Our data indicate that in solution PfGST is present as a tetramer that dissociates into dimers in the presence of glutathione (GSH). Fluorescence spectroscopy shows that in the presence of GSH GST serves as ligandin for parasitotoxic ferriprotoporphyrin IX with a high- and a low-affinity binding site. This is supported by a clear uncompetitive inhibition type. Site-directed mutagenesis studies demonstrate that neither Cys 86 nor Cys 101 contribute to the peroxidase activity of the enzyme, which is thus performed GSH-dependently at the active site. Tyr 9 is responsible for the deprotonation of GSH and Lys 15, but also Gln 71 are involved in GSH binding. We furthermore report the 2.4 A resolution X-ray structure of PfGST cocrystallized with the inhibitor S-hexylglutathione. In comparison with a previously reported structure obtained by crystal soaking, differences occur at the C-terminal end of helix alpha4 and at the S-hexylmoiety of the inhibitor. We furthermore show that, in contrast to previous reports, the antimalarial drug artemisinin is not metabolized by PfGST.
Figures

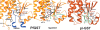

Similar articles
-
X-ray structure of glutathione S-transferase from the malarial parasite Plasmodium falciparum.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13821-6. doi: 10.1073/pnas.2333763100. Epub 2003 Nov 17. Proc Natl Acad Sci U S A. 2003. PMID: 14623980 Free PMC article.
-
Glutathione mediated regulation of oligomeric structure and functional activity of Plasmodium falciparum glutathione S-transferase.BMC Struct Biol. 2007 Oct 17;7:67. doi: 10.1186/1472-6807-7-67. BMC Struct Biol. 2007. PMID: 17941979 Free PMC article.
-
Glutathione S-transferase of the malarial parasite Plasmodium falciparum: characterization of a potential drug target.Biol Chem. 2002 May;383(5):821-30. doi: 10.1515/BC.2002.086. Biol Chem. 2002. PMID: 12108547
-
Glutathione S-transferase from malarial parasites: structural and functional aspects.Methods Enzymol. 2005;401:241-53. doi: 10.1016/S0076-6879(05)01015-3. Methods Enzymol. 2005. PMID: 16399390 Review.
-
Structure, catalytic mechanism, and evolution of the glutathione transferases.Chem Res Toxicol. 1997 Jan;10(1):2-18. doi: 10.1021/tx960072x. Chem Res Toxicol. 1997. PMID: 9074797 Review. No abstract available.
Cited by
-
Compounds structurally related to ellagic acid show improved antiplasmodial activity.Antimicrob Agents Chemother. 2009 Feb;53(2):622-30. doi: 10.1128/AAC.00544-08. Epub 2008 Nov 17. Antimicrob Agents Chemother. 2009. PMID: 19015351 Free PMC article.
-
Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
-
The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases-Part III: In-Silico Molecular Docking Investigations.Molecules. 2016 Oct 19;21(10):1389. doi: 10.3390/molecules21101389. Molecules. 2016. PMID: 27775577 Free PMC article. Review.
-
Is there a role for tau glutathione transferases in tetrapyrrole metabolism and retrograde signalling in plants?Philos Trans R Soc Lond B Biol Sci. 2020 Jun 22;375(1801):20190404. doi: 10.1098/rstb.2019.0404. Epub 2020 May 4. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32362257 Free PMC article.
-
The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures?Antioxid Redox Signal. 2017 Nov 20;27(15):1130-1161. doi: 10.1089/ars.2017.7123. Epub 2017 Jul 19. Antioxid Redox Signal. 2017. PMID: 28540740 Free PMC article. Review.
References
-
- Becker, K., Tilley, L., Vennerstrom, J.L., Roberts, D., Rogerson, S., and Ginsburg, H. 2004. Oxidative stress in malaria parasite-infected erythrocytes: Host–parasite interactions. Int. J. Parasitol. 34: 163–189. - PubMed
-
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. - PubMed
-
- Caccuri, A.M., Lo Bello, M., Nuccetelli, M., Nicotra, M., Rossi, P., Antonini, G., Federici, G., and Ricci, G. 1998. Proton release upon glutathione binding to glutathione transferase P1-1: Kinetic analysis of a multistep glutathione binding process. Biochemistry 37: 3028–3034. - PubMed
-
- Chern, M.K., Wu, T.C., Hsieh, C.H., Chou, C.C., Liu, L.F., Kuan, I.C., Yeh, Y.H., Hsiao, C.D., and Tam, M.F. 2000. Tyr115, gln165 and trp209 contribute to the 1, 2-epoxy-3-(p-nitrophenoxy)propane-conjugating activity of glutathione S-transferase cGSTM1–1. J. Mol. Biol. 300: 1257–1269. - PubMed
-
- Deponte, M. and Becker, K. 2005a. Glutathione S-transferase from malarial parasites—Structural and functional aspects. Methods Enzymol. 401: 240–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

