A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle
- PMID: 16387635
- PMCID: PMC2248602
- DOI: 10.1016/j.neuron.2005.11.032
A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle
Abstract
Quantal size is the postsynaptic response to the release of a single synaptic vesicle and is determined in part by the amount of transmitter within that vesicle. At glutamatergic synapses, the vesicular glutamate transporter (VGLUT) fills vesicles with glutamate. While elevated VGLUT expression increases quantal size, the minimum number of transporters required to fill a vesicle is unknown. In Drosophila DVGLUT mutants, reduced transporter levels lead to a dose-dependent reduction in the frequency of spontaneous quantal release with no change in quantal size. Quantal frequency is not limited by vesicle number or impaired exocytosis. This suggests that a single functional unit of transporter is both necessary and sufficient to fill a vesicle to completion and that vesicles without DVGLUT are empty. Consistent with the presence of empty vesicles, at dvglut mutant synapses synaptic vesicles are smaller, suggesting that vesicle filling and/or transporter level is an important determinant of vesicle size.
Figures
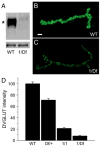
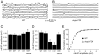
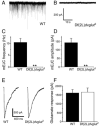
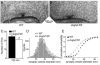

Comment in
-
Vesicular monogamy?Neuron. 2006 Jan 5;49(1):1-2. doi: 10.1016/j.neuron.2005.12.013. Neuron. 2006. PMID: 16387631
References
-
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gunderson V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. - PubMed
-
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. - PubMed
-
- Featherstone DE, Rushton EM, Hilderbrand-Chae M, Phillips AM, Jackson FR, Broadie K. Presynaptic glutamic acid decarboxylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron. 2000;27:71–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

