Dissecting the contribution of diffusion and interactions to the mobility of nuclear proteins
- PMID: 16387760
- PMCID: PMC1386769
- DOI: 10.1529/biophysj.105.071241
Dissecting the contribution of diffusion and interactions to the mobility of nuclear proteins
Abstract
Quantitative characterization of protein interactions under physiological conditions is vital for systems biology. Fluorescence photobleaching/activation experiments of GFP-tagged proteins are frequently used for this purpose, but robust analysis methods to extract physicochemical parameters from such data are lacking. Here, we implemented a reaction-diffusion model to determine the contributions of protein interaction and diffusion on fluorescence redistribution. The model was validated and applied to five chromatin-interacting proteins probed by photoactivation in living cells. We found that very transient interactions are common for chromatin proteins. Their observed mobility was limited by the amount of free protein available for diffusion but not by the short residence time of the bound proteins. Individual proteins thus locally scan chromatin for binding sites, rather than diffusing globally before rebinding at random nuclear positions. By taking the real cellular geometry and the inhomogeneous distribution of binding sites into account, our model provides a general framework to analyze the mobility of fluorescently tagged factors. Furthermore, it defines the experimental limitations of fluorescence perturbation experiments and highlights the need for complementary methods to measure transient biochemical interactions in living cells.
Figures

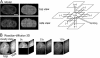



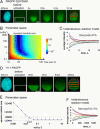
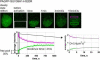
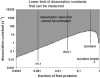
References
-
- Houtsmuller, A. B., S. Rademakers, A. L. Nigg, D. Hoogstraten, J. H. Hoeijmakers, and W. Vermeulen. 1999. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science. 284:958–961. - PubMed
-
- Phair, R. D., and T. Misteli. 2000. High mobility of proteins in the mammalian cell nucleus. Nature. 404:604–609. - PubMed
-
- Misteli, T., A. Gunjan, R. Hock, M. Bustin, and D. T. Brown. 2000. Dynamic binding of histone H1 to chromatin in living cells. Nature. 408:877–881. - PubMed
-
- McNally, J. G., W. G. Muller, D. Walker, R. Wolford, and G. L. Hager. 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 287:1262–1265. - PubMed
-
- Bubulya, P. A., and D. L. Spector. 2004. On the movements of nuclear components in living cells. Exp. Cell Res. 296:4–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

