The structure of phytochrome: a picture is worth a thousand spectra
- PMID: 16387836
- PMCID: PMC1323480
- DOI: 10.1105/tpc.105.038513
The structure of phytochrome: a picture is worth a thousand spectra
Figures
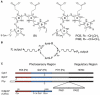
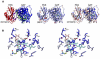

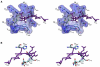
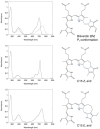
References
-
- Andel, F., Lagarias, J.C., and Mathies, R.A. (1996). Resonance Raman analysis of chromophore structure in the lumi-R photoproduct of phytochrome. Biochemistry 35 15997–16008. - PubMed
-
- Andel, F., Murphy, J.T., Haas, J.A., McDowell, M.T., van der Hoef, I., Lugtenburg, J., Lagarias, J.C., and Mathies, R.A. (2000). Probing the photoreaction mechanism of phytochrome through analysis of resonance Raman vibrational spectra of recombinant analogues. Biochemistry 39 2667–2676. - PubMed
-
- Aravind, L., and Ponting, C.P. (1997). The GAF domain: An evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 22 458–459. - PubMed
-
- Batschauer, A. (2003). Photoreceptors and Light Signaling. (Cambridge, UK: Royal Society of Chemistry).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

