MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis
- PMID: 16387852
- PMCID: PMC1324789
- DOI: 10.1073/pnas.0509463102
MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis
Abstract
We show that MAX1, a specific repressor of vegetative axillary bud outgrowth in Arabidopsis, acts a positive regulator of the flavonoid pathway, including 11 structural genes and the transcription factor An2. Repression of bud outgrowth requires MAX1-dependent flavonoid gene expression. As the flavonoidless state leads to lateral outgrowth in Arabidopsis, our data suggest that a flavonoid-based mechanism regulates axillary bud outgrowth and that this mechanism is under the control of MAX1. Flavonoid gene expression results in the diminished expression of auxin transporters in the bud and stem, and this, in turn, decreases the rate of polar auxin transport. We speculate that MAX1 could repress axillary bud outgrowth via regulating flavonoid-dependent auxin retention in the bud and underlying stem. Because MAX1 is implicated in synthesis of the carotenoid-derived branch regulator(s) from the root, it likely links long-distance signaling with local control of bud outgrowth.
Figures

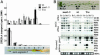

References
-
- Morris, D.A. (1977) Planta 136, 91-96. - PubMed
-
- Cline, M. G. (1994) Physiol. Plant 90, 230-237.
-
- Beveridge, C. A. (2000) Plant Growth Regul. 32, 193-203.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

