Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen
- PMID: 16387855
- PMCID: PMC1326168
- DOI: 10.1073/pnas.0508170103
Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen
Abstract
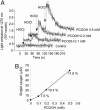
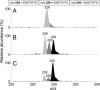
The reaction of hypochlorous acid (HOCl) with hydrogen peroxide is known to generate stoichiometric amounts of singlet molecular oxygen [O2 (1Deltag)]. This study shows that HOCl can also react with linoleic acid hydroperoxide (LAOOH), generating O2 (1Deltag) with a yield of 13 +/- 2% at physiological pH. Characteristic light emission at 1,270 nm, corresponding to O2 (1Deltag) monomolecular decay, was observed when HOCl was reacted with LAOOH or with liposomes containing phosphatidylcholine hydroperoxides, but not with cumene hydroperoxide or tert-butyl hydroperoxide. The generation of O2 (1Deltag) was confirmed by the acquisition of the spectrum of the light emitted in the near-infrared region showing a band with maximum intensity at 1,270 nm and by the observation of the enhancing effect of deuterium oxide and the quenching effect of sodium azide. Mechanistic studies using 18O-labeled linoleic acid hydroperoxide (LA18O18OH) showed that its reaction with HOCl yields 18O-labeled O2 (1Deltag) [18O2 (1Deltag)], demonstrating that the oxygen atoms in O2 (1Deltag) are derived from the hydroperoxide group. Direct analysis of radical intermediates in the reaction of LAOOH with HOCl by continuous-flow electron paramagnetic resonance spectroscopy showed a doublet signal with a g-value of 2.014 and a hyperfine coupling constant from the alpha-hydrogen of a(H) = 4.3 G, indicating the formation of peroxyl radicals. Taken together, our results clearly demonstrate that HOCl reacts with biologically relevant lipid hydroperoxides, generating O2 (1Deltag). In addition, the detection of 18O2 (1Deltag) and peroxyl radicals strongly supports the involvement of a Russell mechanism in the generation of O2 (1Deltag).
Figures







Similar articles
-
Thymine hydroperoxide as a potential source of singlet molecular oxygen in DNA.Free Radic Biol Med. 2009 Aug 15;47(4):401-9. doi: 10.1016/j.freeradbiomed.2009.05.001. Epub 2009 May 6. Free Radic Biol Med. 2009. PMID: 19426799
-
Direct evidence of singlet molecular oxygen [O2(1Deltag)] production in the reaction of linoleic acid hydroperoxide with peroxynitrite.J Am Chem Soc. 2003 Apr 16;125(15):4510-7. doi: 10.1021/ja029262m. J Am Chem Soc. 2003. PMID: 12683821
-
Singlet molecular oxygen generated from lipid hydroperoxides by the russell mechanism: studies using 18(O)-labeled linoleic acid hydroperoxide and monomol light emission measurements.J Am Chem Soc. 2003 May 21;125(20):6172-9. doi: 10.1021/ja029115o. J Am Chem Soc. 2003. PMID: 12785849
-
Biological hydroperoxides and singlet molecular oxygen generation.IUBMB Life. 2007 Apr-May;59(4-5):322-31. doi: 10.1080/15216540701242508. IUBMB Life. 2007. PMID: 17505972 Review.
-
Lipid hydroperoxides as a source of singlet molecular oxygen.Subcell Biochem. 2014;77:3-20. doi: 10.1007/978-94-007-7920-4_1. Subcell Biochem. 2014. PMID: 24374914 Review.
Cited by
-
Superoxide-mediated formation of tyrosine hydroperoxides and methionine sulfoxide in peptides through radical addition and intramolecular oxygen transfer.J Biol Chem. 2009 May 29;284(22):14723-33. doi: 10.1074/jbc.M809396200. Epub 2009 Mar 18. J Biol Chem. 2009. PMID: 19297319 Free PMC article.
-
Trypanosoma brucei Lipophosphoglycan Induces the Formation of Neutrophil Extracellular Traps and Reactive Oxygen Species Burst via Toll-Like Receptor 2, Toll-Like Receptor 4, and c-Jun N-Terminal Kinase Activation.Front Microbiol. 2021 Jul 28;12:713531. doi: 10.3389/fmicb.2021.713531. eCollection 2021. Front Microbiol. 2021. PMID: 34394064 Free PMC article.
-
Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species.Biomolecules. 2019 Jul 5;9(7):258. doi: 10.3390/biom9070258. Biomolecules. 2019. PMID: 31284470 Free PMC article. Review.
-
Biomimetic Aerobic Epoxidation of Alkenes Catalyzed by Cobalt Porphyrin under Ambient Conditions in the Presence of Sunflower Seeds Oil as a Co-Substrate.ACS Omega. 2020 Mar 2;5(10):4890-4899. doi: 10.1021/acsomega.9b03714. eCollection 2020 Mar 17. ACS Omega. 2020. PMID: 32201774 Free PMC article.
-
The Octadecanoids: Synthesis and Bioactivity of 18-Carbon Oxygenated Fatty Acids in Mammals, Bacteria, and Fungi.Chem Rev. 2025 Jan 8;125(1):1-90. doi: 10.1021/acs.chemrev.3c00520. Epub 2024 Dec 16. Chem Rev. 2025. PMID: 39680864 Free PMC article. Review.
References
-
- Harrison, J., Watson, B. & Schultz, J. (1978) FEBS Lett. 92, 327-332. - PubMed
-
- Klebanoff, S. J. (1999) in Inflammation: Basic Principles and Clinical Correlates, eds. Gallin, J. I. & Snyderman, R. (Lippincott, Philadelphia), pp. 721-768.
-
- Hampton, M., Kettle, A. & Winterbourn, C. (1998) Blood 92, 3007-3017. - PubMed
-
- Winterbourn, C. C. & Kettle, A. J. (2000) Free Radical Biol. Med. 29, 403-409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

