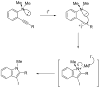
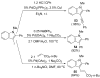
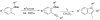Synthesis of 3-iodoindoles by the Pd/Cu-catalyzed coupling of N,N-dialkyl-2-iodoanilines and terminal acetylenes, followed by electrophilic cyclization
- PMID: 16388618
- PMCID: PMC2556959
- DOI: 10.1021/jo051549p
Synthesis of 3-iodoindoles by the Pd/Cu-catalyzed coupling of N,N-dialkyl-2-iodoanilines and terminal acetylenes, followed by electrophilic cyclization
Abstract
[reaction: see text] 3-Iodoindoles have been prepared in excellent yields by coupling terminal acetylenes with N,N-dialkyl-o-iodoanilines in the presence of a Pd/Cu catalyst, followed by an electrophilic cyclization of the resulting N,N-dialkyl-o-(1-alkynyl)anilines using I2 in CH2Cl2. Aryl-, vinylic-, alkyl-, and silyl-substituted terminal acetylenes undergo this process to produce excellent yields of 3-iodoindoles. The reactivity of the carbon-nitrogen bond cleavage during cyclization follows the following order: Me > n-Bu, Me > Ph, and cyclohexyl > Me. Subsequent palladium-catalyzed Sonogashira, Suzuki, and Heck reactions of the resulting 3-iodoindoles proceed smoothly in good yields.
Similar articles
-
Synthesis of 3-sulfenyl- and 3-selenylindoles by the Pd/Cu-catalyzed coupling of N,N-dialkyl-2-iodoanilines and terminal alkynes, followed by n-Bu(4)NI-induced electrophilic cyclization.J Org Chem. 2009 Sep 4;74(17):6802-11. doi: 10.1021/jo9014003. J Org Chem. 2009. PMID: 19663396 Free PMC article.
-
Synthesis of 3-iodoindoles by electrophilic cyclization of N,N-dialkyl-2-(1-alkynyl)anilines.Org Lett. 2004 Mar 18;6(6):1037-40. doi: 10.1021/ol0498996. Org Lett. 2004. PMID: 15012094
-
Highly substituted indole library synthesis by palladium-catalyzed coupling reactions in solution and on a solid support.J Comb Chem. 2009 Sep-Oct;11(5):875-9. doi: 10.1021/cc900057n. J Comb Chem. 2009. PMID: 19746991 Free PMC article.
-
A novel synthetic route to 3-sulfenyl- and 3-selenylindoles by n-Bu4NI-induced electrophilic cyclization.Org Lett. 2009 Jan 1;11(1):173-6. doi: 10.1021/ol8021287. Org Lett. 2009. PMID: 19046068 Free PMC article.
-
Synthesis of heterocyclic compounds through palladium-catalyzed C-H cyclization processes.Chem Pharm Bull (Tokyo). 2013;61(10):987-96. doi: 10.1248/cpb.c13-00420. Chem Pharm Bull (Tokyo). 2013. PMID: 24088691 Review.
Cited by
-
A simple and mild synthesis of 1H-isochromenes and (Z)-1-alkylidene-1,3-dihydroisobenzofurans by the iodocyclization of 2-(1-alkynyl)benzylic alcohols.J Org Chem. 2010 Feb 5;75(3):897-901. doi: 10.1021/jo902333y. J Org Chem. 2010. PMID: 20043652 Free PMC article.
-
Synthesis of 3-sulfenyl- and 3-selenylindoles by the Pd/Cu-catalyzed coupling of N,N-dialkyl-2-iodoanilines and terminal alkynes, followed by n-Bu(4)NI-induced electrophilic cyclization.J Org Chem. 2009 Sep 4;74(17):6802-11. doi: 10.1021/jo9014003. J Org Chem. 2009. PMID: 19663396 Free PMC article.
-
Tandem Cycloisomerization/Suzuki Coupling of Arylethynyl MIDA Boronates.Tetrahedron. 2011 Jun 17;67(24):4306-4312. doi: 10.1016/j.tet.2011.04.011. Tetrahedron. 2011. PMID: 21765556 Free PMC article.
-
Competition studies in alkyne electrophilic cyclization reactions.J Org Chem. 2009 Feb 6;74(3):1141-7. doi: 10.1021/jo802196r. J Org Chem. 2009. PMID: 19105638 Free PMC article.
-
Tuneable access to indole, indolone, and cinnoline derivatives from a common 1,4-diketone Michael acceptor.Beilstein J Org Chem. 2020 Jul 17;16:1722-1731. doi: 10.3762/bjoc.16.144. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32733616 Free PMC article.
References
-
-
For reviews see: Sundberg RJ. Prog Heterocyclic Chem. 1989;1:111.Saxton JE. Nat Prod Rep. 1986;3:357.1987;4:591.1989;6:1.Sundberg RJ. Indoles. Academic Press; New York: 1996. Mori H, Yoshimi N. Mutation Research. 2001;480:201.Gribble GW. J Chem Soc Perkin Trans 1. 2000:1045.Toyota M, Ihara M. Nat Prod Rep. 1998;15:327.Lobo AM, Prabhakar S. J Heterocycl Chem. 2002;39:429.Tokuyama H, Fukuyama T. Kagaku Kogyo. 2001;52:416.Xiong WN, Yang CG, Jiang B. Bioorg Med Chem. 2001;9:1773.Ezquerra J, Pedregal C, Lamas C. J Org Chem. 1996;61:5804.
-
-
- Sonada T, Kawakami M, Ikeda T, Kobayashi S, Taniguchi H. J Chem Soc, Chem Commun. 1976:612.
- Kitamura T, Kobayashi S, Taniguchi H, Hori K. J Am Chem Soc. 1991;113:6240.
- Kitamura T, Takachi T, Kawasato H, Taniguchi H. J Chem Soc, Perkin Trans 1. 1992:1969.
-
- Yao T, Larock RC. Tetrahedron Lett. 2002;43:7401.
- Yao T, Larock RC. J Org Chem. 2003;68:5936. - PubMed
- Oliver MA, Gandour RD. J Org Chem. 1984;49:558.
- Biagetti M, Bellina F, Carpita A, Stabile P, Rossi R. Tetrahedron. 2002;58:5023.
- Rossi R, Carpita A, Bellina F, Stabile P, Mannina L. Tetrahedron. 2003;59:2067.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources