Non-glucan attached proteins of Candida albicans biofilm formed on various surfaces
- PMID: 16389478
- PMCID: PMC4957702
- DOI: 10.1007/s11046-005-0167-2
Non-glucan attached proteins of Candida albicans biofilm formed on various surfaces
Abstract
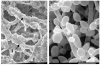
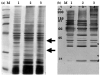
Non-glucan attached proteins of the cell surface and extracellular matrix of Candida albicans biofilms formed on two catheter surfaces and denture acrylic were examined. The SDS-PAGE protein profiles of these proteins compared with that obtained from planktonic yeast cells and germ tubes were generally similar. This observation suggested that this class of biofilm surface proteins is not composed of a unique set of extracellular proteins or that one or a few proteins dominate the non-glucan attached proteins of biofilm. However, differences were observed in the proteins obtained from biofilm formed on one catheter surface and two proteins, Grp2p and ORF19.822p, identified by mass spectrometry following two-dimensional separation. These proteins have previously been associated with drug resistance and their presence or abundance appeared to be influenced by the surface on which the biofilm was formed.
Figures
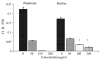

Similar articles
-
[Candida biofilm formation on Provox 2 and Provox Acti Valve voice prosthesis].Otolaryngol Pol. 2010 Nov-Dec;64(6):358-64. doi: 10.1016/S0030-6657(10)70587-4. Otolaryngol Pol. 2010. PMID: 21302502 Polish.
-
Role of dimorphism in the development of Candida albicans biofilms.J Med Microbiol. 1999 Jul;48(7):671-679. doi: 10.1099/00222615-48-7-671. J Med Microbiol. 1999. PMID: 10403418
-
Biofilm development by blastospores and hyphae of Candida albicans on abraded denture acrylic resin surfaces.J Prosthet Dent. 2014 Oct;112(4):988-93. doi: 10.1016/j.prosdent.2014.02.003. Epub 2014 Apr 14. J Prosthet Dent. 2014. PMID: 24726593
-
Genetics and genomics of Candida albicans biofilm formation.Cell Microbiol. 2006 Sep;8(9):1382-91. doi: 10.1111/j.1462-5822.2006.00761.x. Epub 2006 Jul 11. Cell Microbiol. 2006. PMID: 16848788 Review.
-
The role of the Candida biofilm matrix in drug and immune protection.Cell Surf. 2023 Oct 9;10:100111. doi: 10.1016/j.tcsw.2023.100111. eCollection 2023 Dec 15. Cell Surf. 2023. PMID: 37859691 Free PMC article. Review. No abstract available.
Cited by
-
Host contributions to construction of three device-associated Candida albicans biofilms.Infect Immun. 2015 Dec;83(12):4630-8. doi: 10.1128/IAI.00931-15. Epub 2015 Sep 14. Infect Immun. 2015. PMID: 26371129 Free PMC article.
-
Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces.Microbiology (Reading). 2006 Aug;152(Pt 8):2287-2299. doi: 10.1099/mic.0.28959-0. Microbiology (Reading). 2006. PMID: 16849795 Free PMC article.
-
8th ASM conference on Candida and candidiasis: molecular tools provide insights into host-pathogen interactions.Mycopathologia. 2006 Jul;162(1):17-24. doi: 10.1007/s11046-006-0033-x. Mycopathologia. 2006. PMID: 16830187 No abstract available.
-
Analysis of Pathogenic Bacterial and Yeast Biofilms Using the Combination of Synchrotron ATR-FTIR Microspectroscopy and Chemometric Approaches.Molecules. 2021 Jun 25;26(13):3890. doi: 10.3390/molecules26133890. Molecules. 2021. PMID: 34202224 Free PMC article.
-
A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing.Nat Protoc. 2008;3(9):1494-500. doi: 10.1038/nport.2008.141. Nat Protoc. 2008. PMID: 18772877 Free PMC article.
References
-
- Elving GJ, van der Mei HC, Busscher HJ, van Weissenbruch R, Albers FW. Comparison of the microbial composition of voice prosthesis biofilms from patients requiring frequent versus infrequent replacement. Ann Otol Rhinol Laryngol. 2002;111(3 Pt 1):200–203. - PubMed
-
- Gottlieb K, Mobarhan S. Review: microbiology of the gastrostomy tube. J Am Coll Nutr. 1994;13(4):311–313. - PubMed
-
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J. 1998;43(3):160–166. - PubMed
-
- Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J. 1998;185(8):380–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

