Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability
- PMID: 16390845
- PMCID: PMC2803408
- DOI: 10.1093/aob/mcj604
Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability
Erratum in
- Ann Bot (Lond). 2006 Nov;98(5):1115
Abstract
Background and aims: The basic regulatory mechanisms that control lateral root (LR) initiation are still poorly understood. An attempt is made to characterize the pattern and timing of LR initiation, to define a developmental window in which LR initiation takes place and to address the question of whether LR initiation is predictable.
Methods: The spatial patterning of LRs and LR primordia (LRPs) on cleared root preparations were characterized. New measures of LR and LRP densities (number of LRs and/or LRPs divided by the length of the root portions where they are present) were introduced and illustrate the shortcomings of the more customarily used measure through a comparative analysis of the mutant aux1-7. The enhancer trap line J0121 was used to monitor LR initiation in time-lapse experiments and a plasmolysis-based method was developed to determine the number of pericycle cells between successive LRPs.
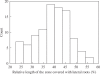
Key results: LRP initiation occurred strictly acropetally and no de novo initiation events were found between already developed LRs or LRPs. However, LRPs did not become LRs in a similar pattern. The longitudinal spacing of lateral organs was variable and the distance between lateral organs was proportional to the number of cells and the time between initiations of successive LRPs. There was a strong tendency towards alternation in LR initiation between the two pericycle cell files adjacent to the protoxylem poles. LR density increased with time due to the emergence of slowly developing LRPs and appears to be unique for individual Arabidopsis accessions.
Conclusions: In Arabidopsis there is a narrow developmental window for LR initiation, and no specific cell-count or distance-measuring mechanisms have been found that determine the site of successive initiation events. Nevertheless, the branching density and lateral organ density (density of LRs and LRPs) are accession-specific, and based on the latter density the average distance between successive LRs can be predicted.
Figures

Similar articles
-
Genetic and Phenotypic Analysis of Lateral Root Development in Arabidopsis thaliana.Methods Mol Biol. 2018;1761:47-75. doi: 10.1007/978-1-4939-7747-5_4. Methods Mol Biol. 2018. PMID: 29525948
-
Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis.J Exp Bot. 2015 Aug;66(15):4759-68. doi: 10.1093/jxb/erv252. Epub 2015 May 27. J Exp Bot. 2015. PMID: 26019251 Free PMC article.
-
Lateral root formation and the multiple roles of auxin.J Exp Bot. 2018 Jan 4;69(2):155-167. doi: 10.1093/jxb/erx223. J Exp Bot. 2018. PMID: 28992266 Review.
-
Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis.Plant J. 2005 Nov;44(3):382-95. doi: 10.1111/j.1365-313X.2005.02537.x. Plant J. 2005. PMID: 16236149
-
Shaping root architecture: towards understanding the mechanisms involved in lateral root development.Biol Direct. 2024 Oct 2;19(1):87. doi: 10.1186/s13062-024-00535-5. Biol Direct. 2024. PMID: 39358783 Free PMC article. Review.
Cited by
-
Neutral red as a probe for confocal laser scanning microscopy studies of plant roots.Ann Bot. 2006 Jun;97(6):1127-38. doi: 10.1093/aob/mcl045. Epub 2006 Mar 6. Ann Bot. 2006. PMID: 16520341 Free PMC article.
-
Intercellular communication during plant development.Plant Cell. 2011 Mar;23(3):855-64. doi: 10.1105/tpc.111.082982. Epub 2011 Mar 8. Plant Cell. 2011. PMID: 21386031 Free PMC article. Review.
-
Auxin Modulated Initiation of Lateral Roots Is Linked to Pericycle Cell Length in Maize.Front Plant Sci. 2019 Jan 24;10:11. doi: 10.3389/fpls.2019.00011. eCollection 2019. Front Plant Sci. 2019. PMID: 30733725 Free PMC article.
-
Arabidopsis homolog of trithorax1 (ATX1) is required for cell production, patterning, and morphogenesis in root development.J Exp Bot. 2014 Dec;65(22):6373-84. doi: 10.1093/jxb/eru355. Epub 2014 Sep 9. J Exp Bot. 2014. PMID: 25205583 Free PMC article.
-
Priming and positioning of lateral roots in Arabidopsis. An approach for an integrating concept.J Exp Bot. 2016 Mar;67(5):1411-20. doi: 10.1093/jxb/erv541. Epub 2015 Dec 27. J Exp Bot. 2016. PMID: 26712828 Free PMC article.
References
-
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. 2005. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany 56: 1535–1544. - PubMed
-
- Barlow PW, Adam JS. 1988. The position and growth of lateral roots on cultured root axes of tomato, Lycopersicon esculentum (Solanaceae). Plant Systematics and Evolution 158: 141–154.
-
- Barlow PW, Volkmann D, Baluška F. 2004. Polarity in roots. In: Lindsey K, ed. Polarity in plants. Oxford: Blackwell Publishing, 192–241.
-
- Blakely LM, Durham M, Evans TA, Blakely RM. 1982. Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Botanical Gazette 143: 341–352.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

