Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis
- PMID: 16390935
- PMCID: PMC2118093
- DOI: 10.1084/jem.20050433
Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis
Abstract
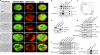
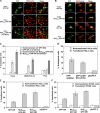
The mechanisms by which glucocorticoid receptor (GR) mediates glucocorticoid (GC)-induced apoptosis are unknown. We studied the role of mitochondrial GR in this process. Dexamethasone induces GR translocation to the mitochondria in GC-sensitive, but not in GC-resistant, T cell lines. In contrast, nuclear GR translocation occurs in all cell types. Thymic epithelial cells, which cause apoptosis of the PD1.6 T cell line in a GR-dependent manner, induce GR translocation to the mitochondria, but not to the nucleus, suggesting a role for mitochondrial GR in eliciting apoptosis. This hypothesis is corroborated by the finding that a GR variant exclusively expressed in the mitochondria elicits apoptosis of several cancer cell lines. A putative mitochondrial localization signal was defined to amino acids 558-580 of human GR, which lies within the NH2-terminal part of the ligand-binding domain. Altogether, our data show that mitochondrial and nuclear translocations of GR are differentially regulated, and that mitochondrial GR translocation correlates with susceptibility to GC-induced apoptosis.
Figures





Similar articles
-
The glucocorticoid receptor mediates the thymic epithelial cell-induced apoptosis of CD4+8+ thymic lymphoma cells.Cell Immunol. 2004 Jan;227(1):12-23. doi: 10.1016/j.cellimm.2004.01.005. Cell Immunol. 2004. PMID: 15051511
-
Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis.Int Immunol. 2009 Nov;21(11):1269-76. doi: 10.1093/intimm/dxp093. Epub 2009 Sep 7. Int Immunol. 2009. PMID: 19737783
-
Resistance to glucocorticoid-induced apoptosis in human T-cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression.Cancer Res. 1996 Nov 1;56(21):5033-8. Cancer Res. 1996. PMID: 8895760
-
Emerging pathways of non-genomic glucocorticoid (GC) signalling in T cells.Immunobiology. 2010 Jul;215(7):521-6. doi: 10.1016/j.imbio.2009.10.003. Epub 2009 Nov 10. Immunobiology. 2010. PMID: 19906460 Review.
-
Modulatory effects of L-carnitine on glucocorticoid receptor activity.Ann N Y Acad Sci. 2004 Nov;1033:147-57. doi: 10.1196/annals.1320.014. Ann N Y Acad Sci. 2004. PMID: 15591012 Review.
Cited by
-
The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats.Int J Biochem Cell Biol. 2009 Nov;41(11):2181-8. doi: 10.1016/j.biocel.2009.04.001. Epub 2009 Apr 9. Int J Biochem Cell Biol. 2009. PMID: 19782950 Free PMC article.
-
Brain mitochondrial drug delivery: influence of drug physicochemical properties.Pharm Res. 2011 Nov;28(11):2833-47. doi: 10.1007/s11095-011-0532-4. Epub 2011 Jul 28. Pharm Res. 2011. PMID: 21796482 Free PMC article.
-
Mitochondriotropic Derivative of Ethyl Ferulate, a Dietary Phenylpropanoid, Exhibits Enhanced Cytotoxicity in Cancer Cells via Mitochondrial Superoxide-Mediated Activation of JNK and AKT Signalling.Appl Biochem Biotechnol. 2023 Mar;195(3):2057-2076. doi: 10.1007/s12010-022-04252-5. Epub 2022 Nov 21. Appl Biochem Biotechnol. 2023. PMID: 36409426
-
A role for bcl-2 in notch1-dependent transcription in thymic lymphoma cells.Adv Hematol. 2012;2012:435241. doi: 10.1155/2012/435241. Epub 2012 Jan 26. Adv Hematol. 2012. PMID: 22319533 Free PMC article.
-
Increased Expression of the Mitochondrial Glucocorticoid Receptor Enhances Tumor Aggressiveness in a Mouse Xenograft Model.Int J Mol Sci. 2023 Feb 13;24(4):3740. doi: 10.3390/ijms24043740. Int J Mol Sci. 2023. PMID: 36835152 Free PMC article.
References
-
- Haarman, E.G., G.J. Kaspers, and A.J. Veerman. 2003. Glucocorticoid resistance in childhood leukaemia: mechanisms and modulation. Br. J. Haematol. 120:919–929. - PubMed
-
- Tissing, W.J., J.P. Meijerink, M.L. den Boer, and R. Pieters. 2003. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 17:17–25. - PubMed
-
- Greenstein, S., K. Ghias, N.L. Krett, and S.T. Rosen. 2002. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin. Cancer Res. 8:1681–1694. - PubMed
-
- Lu, N.Z., and J.A. Cidlowski. 2004. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann. NY Acad. Sci. 1024:102–123. - PubMed
-
- Schaaf, M.J., and J.A. Cidlowski. 2002. Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 83:37–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

