Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection
- PMID: 16390939
- PMCID: PMC2118084
- DOI: 10.1084/jem.20051836
Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection
Abstract
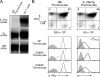
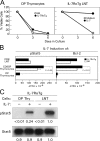
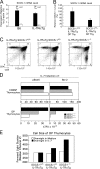
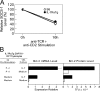
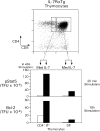
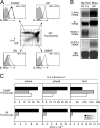
Death by neglect requires that CD4+8+ double-positive (DP) thymocytes avoid cytokine-mediated survival signals, which is presumably why DP thymocytes normally extinguish IL-7R gene expression. We report that DP thymocytes before positive selection (preselection DP thymocytes) fail to transduce IL-7 signals even when they express high levels of transgenic IL-7R on their surface, because IL-7R signal transduction is actively suppressed in preselection DP thymocytes by suppressor of cytokine signaling (SOCS)-1. SOCS-1 is highly expressed in preselection DP thymocytes, but it is down-regulated by T cell receptor-mediated positive selection signals. Interestingly, we found that the uniquely small cell volume of DP thymocytes is largely the result of absent IL-7 signaling in preselection DP thymocytes. We also report that, contrary to current concepts, preselection DP thymocytes express high levels of endogenously encoded IL-4Rs. However, their ability to transduce cytokine signals is similarly suppressed by SOCS-1. Thus, despite high surface expression of transgenic or endogenous cytokine receptors, cytokine signal transduction is actively suppressed in preselection DP thymocytes until it is restored by positive selection.
Figures

References
-
- Fowlkes, B.J., and E. Schweighoffer. 1995. Positive selection of T cells. Curr. Opin. Immunol. 7:188–195. - PubMed
-
- Jameson, S.C., K.A. Hogquist, and M.J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93–126. - PubMed
-
- Cao, X., E.W. Shores, J. Hu-Li, M.R. Anver, B.L. Kelsall, S.M. Russell, J. Drago, M. Noguchi, A. Grinberg, E.T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 2:223–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

