Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences
- PMID: 16390999
- PMCID: PMC2063536
- DOI: 10.1083/jcb.200508085
Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences
Abstract
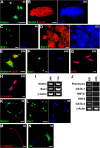
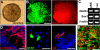
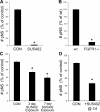
The mechanisms governing the emergence of the earliest mammalian neural cells during development remain incompletely characterized. A default mechanism has been suggested to underlie neural fate acquisition; however, an instructive process has also been proposed. We used mouse embryonic stem (ES) cells to explore the fundamental issue of how an uncommitted, pluripotent mammalian cell will self-organize in the absence of extrinsic signals and what cellular fate will result. To assess this default state, ES cells were placed in conditions that minimize external influences. Individual ES cells were found to rapidly transition directly into neural cells, a process shown to be independent of suggested instructive factors (e.g., fibroblast growth factors). Further, we provide evidence that the default neural identity is that of a primitive neural stem cell (NSC). The exiguous conditions used to reveal the default state were found to present primitive NSCs with a survival challenge (limiting their persistence and proliferation), which could be mitigated by survival factors or genetic interference with apoptosis.
Figures




References
-
- Bainter, J.J., A. Boos, and K.L. Kroll. 2001. Neural induction takes a transcriptional twist. Dev. Dyn. 222:315–327. - PubMed
-
- Beddington, R.S., and E.J. Robertson. 1989. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 105:733–737. - PubMed
-
- Cai, J., J. Yang, and D.P. Jones. 1998. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim. Biophys. Acta. 1366:139–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

