Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis
- PMID: 16391000
- PMCID: PMC2063538
- DOI: 10.1083/jcb.200508001
Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis
Abstract
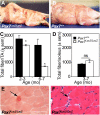
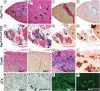
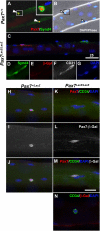
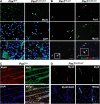
We assessed viable Pax7(-/-) mice in 129Sv/J background and observed reduced growth and marked muscle wasting together with a complete absence of functional satellite cells. Acute injury resulted in an extreme deficit in muscle regeneration. However, a small number of regenerated myofibers were detected, suggesting the presence of residual myogenic cells in Pax7-deficient muscle. Rare Pax3(+)/MyoD+ myoblasts were recovered from Pax7(-/-) muscle homogenates and cultures of myofiber bundles but not from single myofibers free of interstitial tissues. Finally, we identified Pax3+ cells in the muscle interstitial environment and demonstrated that they coexpressed MyoD during regeneration. Sublaminar satellite cells in hind limb muscle did not express detectable levels of Pax3 protein or messenger RNA. Therefore, we conclude that interstitial Pax3+ cells represent a novel myogenic population that is distinct from the sublaminar satellite cell lineage and that Pax7 is essential for the formation of functional myogenic progenitors from sublaminar satellite cells.
Figures


Similar articles
-
Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells.J Cell Biol. 2006 Jan 2;172(1):91-102. doi: 10.1083/jcb.200508044. Epub 2005 Dec 27. J Cell Biol. 2006. PMID: 16380438 Free PMC article.
-
Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis.Int J Dev Biol. 2006;50(1):47-54. doi: 10.1387/ijdb.052111dh. Int J Dev Biol. 2006. PMID: 16323077
-
Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development.Nat Cell Biol. 2010 Mar;12(3):257-66. doi: 10.1038/ncb2025. Epub 2010 Jan 31. Nat Cell Biol. 2010. PMID: 20118923
-
Skeletal muscle progenitor cells and the role of Pax genes.C R Biol. 2007 Jun-Jul;330(6-7):530-3. doi: 10.1016/j.crvi.2007.03.015. Epub 2007 Jun 13. C R Biol. 2007. PMID: 17631448 Review.
-
PAX3 and PAX7 as upstream regulators of myogenesis.Semin Cell Dev Biol. 2015 Aug;44:115-25. doi: 10.1016/j.semcdb.2015.09.017. Epub 2015 Sep 28. Semin Cell Dev Biol. 2015. PMID: 26424495 Review.
Cited by
-
Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells.Mol Cell Biol. 2012 Jun;32(12):2300-11. doi: 10.1128/MCB.06753-11. Epub 2012 Apr 9. Mol Cell Biol. 2012. PMID: 22493066 Free PMC article.
-
Resistance training induced increase in muscle fiber size in young and older men.Eur J Appl Physiol. 2013 Mar;113(3):641-50. doi: 10.1007/s00421-012-2466-x. Epub 2012 Aug 17. Eur J Appl Physiol. 2013. PMID: 22898716 Clinical Trial.
-
Sdf-1 (CXCL12) induces CD9 expression in stem cells engaged in muscle regeneration.Stem Cell Res Ther. 2015 Mar 24;6(1):46. doi: 10.1186/s13287-015-0041-1. Stem Cell Res Ther. 2015. PMID: 25890097 Free PMC article.
-
Myogenic exosome miR-140-5p modulates skeletal muscle regeneration and injury repair by regulating muscle satellite cells.Aging (Albany NY). 2024 Feb 29;16(5):4609-4630. doi: 10.18632/aging.205617. Epub 2024 Feb 29. Aging (Albany NY). 2024. PMID: 38428405 Free PMC article.
-
Intrinsic changes and extrinsic influences of myogenic stem cell function during aging.Stem Cell Rev. 2007 Fall;3(3):226-37. doi: 10.1007/s12015-007-9000-2. Stem Cell Rev. 2007. PMID: 17917136 Review.
References
-
- Ben-Yair, R., and C. Kalcheim. 2005. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development. 132:689–701. - PubMed
-
- Bittner, R.E., C. Schofer, K. Weipoltshammer, S. Ivanova, B. Streubel, E. Hauser, M. Freilinger, H. Hoger, A. Elbe-Burger, and F. Wachtler. 1999. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat. Embryol. (Berl.). 199:391–396. - PubMed
-
- Blau, H.M., T.R. Brazelton, and J.M. Weimann. 2001. The evolving concept review of a stem cell: entity or function? Cell. 105:829–841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

