Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation
- PMID: 16391003
- PMCID: PMC2063542
- DOI: 10.1083/jcb.200506184
Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation
Abstract
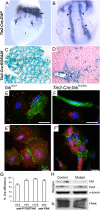
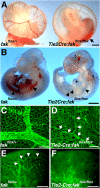
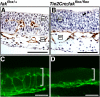
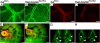
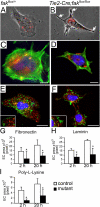
Morphogenesis of a vascular network requires dynamic vessel growth and regression. To investigate the cellular mechanism underlying this process, we deleted focal adhesion kinase (FAK), a key signaling mediator, in endothelial cells (ECs) using Tie2-Cre mice. Targeted FAK depletion occurred efficiently early in development, where mutants exhibited a distinctive and irregular vasculature, resulting in hemorrhage and lethality between embryonic day (e) 10.5 and 11.5. Capillaries and intercapillary spaces in yolk sacs were dilated before any other detectable abnormalities at e9.5, and explants demonstrate that the defects resulted from the loss of FAK and not from organ failure. Time-lapse microscopy monitoring EC behavior during vascular formation in explants revealed no apparent decrease in proliferation or migration but revealed increases in cell retraction and death leading to reduced vessel growth and increased vessel regression. Consistent with this phenotype, ECs derived from mutant embryos exhibited aberrant lamellipodial extensions, altered actin cytoskeleton, and nonpolarized cell movement. This study reveals that FAK is crucial for vascular morphogenesis and the regulation of EC survival and morphology.
Figures
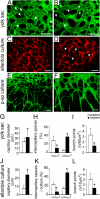
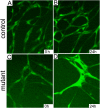
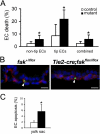
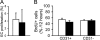
References
-
- Alavi, A., J.D. Hood, R. Frausto, D.G. Stupack, and D.A. Cheresh. 2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 301:94–96. - PubMed
-
- Ausprunk, D.H., and J. Folkman. 1977. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 14:53–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

