Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi
- PMID: 16391013
- PMCID: PMC1457097
- DOI: 10.1182/blood-2005-10-4168
Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi
Abstract
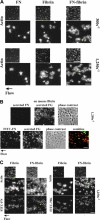
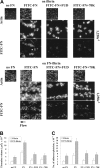
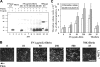
To learn how plasma fibronectin stabilizes platelet-rich thrombi in injured mesenteric arterioles of mice, we studied the impact of plasma fibronectin on platelet thrombus formation ex vivo in a parallel flow chamber. Thrombi were greater on surfaces coated with fibrin cross-linked to fibronectin by activated factor XIII than on surfaces coated with fibrin lacking cross-linked fibronectin or with fibronectin alone. Platelet thrombi were even greater when plasma fibronectin was perfused with platelets, resulting in deposition of the perfused fibronectin in platelet thrombi. The effect of perfused fibronectin on thrombogenesis was lost if fibronectin deposition was blocked by coperfusion with the N-terminal 70-kDa fragment of fibronectin or a peptide based on the functional upstream domain of protein F1 of Streptococcus pyogenes. Increases in thrombus formation were dependent on a platelet activator such as lysophosphatidic acid, amount of fibronectin cross-linked to fibrin, and concentration of fibronectin in the perfusate. The dependency of fibronectin concentration extended into the range of fibronectin concentrations associated with increased risk of coronary artery disease. At such concentrations, the 2 mechanisms for insolubilization of plasma fibronectin-cross-linking to fibrin and assembly by adherent and aggregating platelets-synergize to result in many-fold enhancement of platelet thrombus formation.
Figures


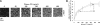
References
-
- Mosher D. Fibronectin. San Diego, CA: Academic Press; 1989.
-
- Hynes R. Fibronectins. New York, NY: Springer-Verlag; 1990.
-
- Mosher DF. Fibronectin. Prog Hemost Thromb. 1980;5: 111-151. - PubMed
-
- Ginsberg MH, Plow EF. Fibronectin: a contender in platelet adhesive function. Academic Press, Harcourt Brace Jovanovich, Publishers; San Diego, CA: 1989.
-
- Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7: 324-330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

