TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens
- PMID: 16391018
- PMCID: PMC1895778
- DOI: 10.1182/blood-2005-06-2259
TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens
Abstract
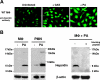
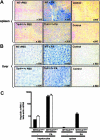
Hepcidin is an antimicrobial peptide secreted by the liver during inflammation that plays a central role in mammalian iron homeostasis. Here we demonstrate the endogenous expression of hepcidin by macrophages and neutrophils in vitro and in vivo. These myeloid cell types produced hepcidin in response to bacterial pathogens in a toll-like receptor 4 (TLR4)-dependent fashion. Conversely, bacterial stimulation of macrophages triggered a TLR4-dependent reduction in the iron exporter ferroportin. In vivo, intraperitoneal challenge with Pseudomonas aeruginosa induced TLR4-dependent hepcidin expression and iron deposition in splenic macrophages, findings mirrored in subcutaneous infection with group A Streptococcus where hepcidin induction was further observed in neutrophils migrating to the tissue site of infection. Hepcidin expression in cultured hepatocytes or in the livers of mice infected with bacteria was independent of TLR4, suggesting the TLR4-hepcidin pathway is restricted to myeloid cell types. Our findings identify endogenous myeloid cell hepcidin production as a previously unrecognized component of the host response to bacterial pathogens.
Figures

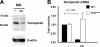
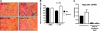


References
-
- Ganz T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18: 171-182. - PubMed
-
- Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000; 480: 147-150. - PubMed
-
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276: 7806-7810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

