Production of recombinant and tagged proteins in the hyperthermophilic archaeon Sulfolobus solfataricus
- PMID: 16391031
- PMCID: PMC1352248
- DOI: 10.1128/AEM.72.1.102-111.2006
Production of recombinant and tagged proteins in the hyperthermophilic archaeon Sulfolobus solfataricus
Abstract
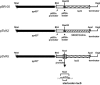
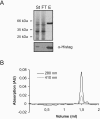
Many systems are available for the production of recombinant proteins in bacterial and eukaryotic model organisms, which allow us to study proteins in their native hosts and to identify protein-protein interaction partners. In contrast, only a few transformation systems have been developed for archaea, and no system for high-level gene expression existed for hyperthermophilic organisms. Recently, a virus-based shuttle vector with a reporter gene was developed for the crenarchaeote Sulfolobus solfataricus, a model organism of hyperthermophilic archaea that grows optimally at 80 degrees C (M. Jonuscheit, E. Martusewitsch, K. M. Stedman, and C. Schleper, Mol. Microbiol. 48:1241-1252, 2003). Here we have refined this system for high-level gene expression in S. solfataricus with the help of two different promoters, the heat-inducible promoter of the major chaperonin, thermophilic factor 55, and the arabinose-inducible promoter of the arabinose-binding protein AraS. Functional expression of heterologous and homologous genes was demonstrated, including production of the cytoplasmic sulfur oxygenase reductase from Acidianus ambivalens, an Fe-S protein of the ABC class from S. solfataricus, and two membrane-associated ATPases potentially involved in the secretion of proteins. Single-step purification of the proteins was obtained via fused His or Strep tags. To our knowledge, these are the first examples of the application of an expression vector system to produce large amounts of recombinant and also tagged proteins in a hyperthermophilic archaeon.
Figures

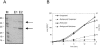


References
-
- Albers, S. V., and A. J. Driessen. 2005. Analysis of ATPases of putative secretion operons in the thermoacidophilic archaeon Sulfolobus solfataricus. Microbiology 151:763-773. - PubMed
-
- Allers, T., and M. Mevarech. 2005. Archaeal genetics—the third way. Nat. Rev. Genet. 6:58-73. - PubMed
-
- Aravalli, R. N., and R. A. Garrett. 1997. Shuttle vectors for hyperthermophilic archaea. Extremophiles 1:183-191. - PubMed
-
- Bell, S. D., C. H. Botting, B. N. Wardleworth, S. P. Jackson, and M. F. White. 2002. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296:148-151. - PubMed
-
- Benelli, D., E. Maone, and P. Londei. 2003. Two different mechanisms for ribosome/mRNA interaction in archaeal translation initiation. Mol. Microbiol. 50:635-643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

