Multicopy integration and expression of heterologous genes in Methylobacterium extorquens ATCC 55366
- PMID: 16391115
- PMCID: PMC1352282
- DOI: 10.1128/AEM.72.1.753-759.2006
Multicopy integration and expression of heterologous genes in Methylobacterium extorquens ATCC 55366
Abstract
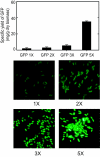
High-level expression of chromosomally integrated genes in Methylobacterium extorquens ATCC 55366 was achieved under the control of the strong M. extorquens AM1 methanol dehydrogenase promoter (PmxaF) using the mini-Tn7 transposon system. Stable maintenance and expression of the integrated genes were obtained in the absence of antibiotic selective pressure. Furthermore, using this technology, a multicopy integration protocol for M. extorquens was also developed. Chromosomal integration of one to five copies of the gene encoding the green fluorescent protein (gfp) was achieved. The multicopy-based expression system permitted expression of a preset number of gene copies. A unique specific Tn7 integration locus in the chromosome of M. extorquens, known as the Tn7 attachment site (attTn7 site), was identified. This single attTn7 site was identified in an intergenic region between glmS, which encodes the essential enzyme glucosamine-6-phosphate synthetase, and dhaT, which encodes 1,3-propanediol dehydrogenase. The fact that the integration event is site specific and the fact that the attTn7 site is a noncoding region of the chromosome make the mini-Tn7 transposon system very useful for insertion of target genes and subsequent expression. In all transformants tested, expression and segregation of the transforming gene were stable without generation of secondary mutations in the host. In this paper, we describe single and multicopy chromosome integration and stable expression of heterologous genes (bgl [beta-galactosidase], est [esterase], and gfp [green fluorescent protein]) in M. extorquens.
Figures



Similar articles
-
Bestowing inducibility on the cloned methanol dehydrogenase promoter (PmxaF) of Methylobacterium extorquens by applying regulatory elements of Pseudomonas putida F1.Appl Environ Microbiol. 2006 Dec;72(12):7723-9. doi: 10.1128/AEM.02002-06. Epub 2006 Oct 13. Appl Environ Microbiol. 2006. PMID: 17041156 Free PMC article.
-
Multicopy integration of mini-Tn7 transposons into selected chromosomal sites of a Salmonella vaccine strain.Microb Biotechnol. 2015 Jan;8(1):177-87. doi: 10.1111/1751-7915.12187. Epub 2014 Dec 9. Microb Biotechnol. 2015. PMID: 25488129 Free PMC article.
-
mini-Tn7 insertion in bacteria with secondary, non-glmS-linked attTn7 sites: example Proteus mirabilis HI4320.Nat Protoc. 2006;1(1):170-8. doi: 10.1038/nprot.2006.26. Nat Protoc. 2006. PMID: 17406229
-
Modified Tn7 transposon vectors for controlled chromosomal gene expression.Appl Environ Microbiol. 2024 Oct 23;90(10):e0155624. doi: 10.1128/aem.01556-24. Epub 2024 Sep 18. Appl Environ Microbiol. 2024. PMID: 39291982 Free PMC article.
-
Methylobacterium extorquens: methylotrophy and biotechnological applications.Appl Microbiol Biotechnol. 2015 Jan;99(2):517-34. doi: 10.1007/s00253-014-6240-3. Epub 2014 Nov 30. Appl Microbiol Biotechnol. 2015. PMID: 25432674 Review.
Cited by
-
Bestowing inducibility on the cloned methanol dehydrogenase promoter (PmxaF) of Methylobacterium extorquens by applying regulatory elements of Pseudomonas putida F1.Appl Environ Microbiol. 2006 Dec;72(12):7723-9. doi: 10.1128/AEM.02002-06. Epub 2006 Oct 13. Appl Environ Microbiol. 2006. PMID: 17041156 Free PMC article.
-
Engineering a Fusion Protein Biomaterial Based on SpyTag/SpyCatcher Bioconjugation of Elastin and Collagen Synthetic Proteins.ACS Omega. 2025 Apr 16;10(16):16245-16256. doi: 10.1021/acsomega.4c10313. eCollection 2025 Apr 29. ACS Omega. 2025. PMID: 40321559 Free PMC article.
-
GFP tagging of Brucella melitensis Rev1 allows the identification of vaccinated sheep.Transbound Emerg Dis. 2019 Jan;66(1):505-516. doi: 10.1111/tbed.13053. Epub 2018 Nov 26. Transbound Emerg Dis. 2019. PMID: 30375177 Free PMC article.
-
Production of an insecticidal crystal protein from Bacillus thuringiensis by the methylotroph Methylobacterium extorquens.Appl Environ Microbiol. 2008 Aug;74(16):5178-82. doi: 10.1128/AEM.00598-08. Epub 2008 Jun 13. Appl Environ Microbiol. 2008. PMID: 18552184 Free PMC article.
-
Phage Mu-driven two-plasmid system for integration of recombinant DNA in the Methylophilus methylotrophus genome.Appl Microbiol Biotechnol. 2008 Nov;81(1):191-200. doi: 10.1007/s00253-008-1696-7. Epub 2008 Sep 27. Appl Microbiol Biotechnol. 2008. PMID: 18820908 Free PMC article.
References
-
- Amaratunga, K., P. M. Goodwin, D. O'Connor, and C. Anthony. 1997. The methanol oxidation genes mxaFJGIR(S)ACKLD in Methylobacterium extorquens. FEMS Microbiol. Lett. 146:31-38. - PubMed
-
- Anthony, C. 1993. Methanol dehydrogenase in Gram-negative bacteria, p. 17-45. In V. Davidson (ed.), Principles and applications of quinoproteins. Dekker, New York, N.Y.
-
- Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7 system for the single-copy insertion of cloned genes into chromosomes of Gram-negative bacteria. Gene 109:167-168. - PubMed
-
- Béland, M., D. Bourque, M. Perrier, and C. B. Míguez. 2004. On-line estimation of stoichiometric growth parameters for Methylotrophic extorquens, p. 49-54. In 9th International Symposium on Computer Applications in Biotechnology (CAB9). Elsevier, Amsterdam, The Netherlands.
-
- Bélanger, L., M. M. Figueira, D. Bourque, L. Morel, M. Béland, L. Laramée, D. Groleau, and C. B. Míguez. 2004. Production of heterologous protein by Methylobacterium extorquens in high cell density fermentation. FEMS Microbiol. Lett. 231:197-204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

