Immobilization of cells with surface-displayed chitin-binding domain
- PMID: 16391137
- PMCID: PMC1352180
- DOI: 10.1128/AEM.72.1.927-931.2006
Immobilization of cells with surface-displayed chitin-binding domain
Abstract
To explore chitin-binding domain (ChBD)-based cell immobilization, a tripartite gene fusion consisting of an in-frame fusion of ChBD to lpp and ompA was constructed and expressed in Escherichia coli. ChBD-displayed cells exhibited highly specific and stable binding to chitin within a wide range of pHs (5 to 8) and temperatures (15 to 37 degrees C). These results illustrate the promising use of this approach for engineering applications.
Figures


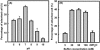
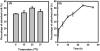
Similar articles
-
Surface display of bacterial metallothioneins and a chitin binding domain on Escherichia coli increase cadmium adsorption and cell immobilization.Appl Biochem Biotechnol. 2012 Jun;167(3):462-73. doi: 10.1007/s12010-012-9684-x. Epub 2012 May 5. Appl Biochem Biotechnol. 2012. PMID: 22562496
-
Identification and characterization of the three chitin-binding domains within the multidomain chitinase Chi92 from Aeromonas hydrophila JP101.Appl Environ Microbiol. 2001 Nov;67(11):5100-6. doi: 10.1128/AEM.67.11.5100-5106.2001. Appl Environ Microbiol. 2001. PMID: 11679332 Free PMC article.
-
Swapping the chitin-binding domain in Bacillus chitinases improves the substrate binding affinity and conformational stability.Mol Biosyst. 2010 Aug;6(8):1492-502. doi: 10.1039/b923048c. Epub 2010 May 26. Mol Biosyst. 2010. PMID: 20502809
-
Lipoprotein trafficking in Escherichia coli.Arch Microbiol. 2004 Sep;182(1):1-6. doi: 10.1007/s00203-004-0682-4. Epub 2004 Jun 24. Arch Microbiol. 2004. PMID: 15221203 Review.
-
The expression of recombinant proteins on the external surface of Escherichia coli. Biotechnological applications.Ann N Y Acad Sci. 1994 Nov 30;745:372-82. doi: 10.1111/j.1749-6632.1994.tb44389.x. Ann N Y Acad Sci. 1994. PMID: 7832524 Review.
Cited by
-
Immobilization of Pichia pastoris cells containing alcohol oxidase activity.Iran J Microbiol. 2011 Dec;3(4):210-5. Iran J Microbiol. 2011. PMID: 22530090 Free PMC article.
-
Surface display of PbrR on Escherichia coli and evaluation of the bioavailability of lead associated with engineered cells in mice.Sci Rep. 2018 Apr 9;8(1):5685. doi: 10.1038/s41598-018-24134-3. Sci Rep. 2018. PMID: 29632327 Free PMC article.
-
N-terminal fusion of a hyperthermophilic chitin-binding domain to xylose isomerase from Thermotoga neapolitana enhances kinetics and thermostability of both free and immobilized enzymes.Biotechnol Prog. 2010 Jul-Aug;26(4):993-1000. doi: 10.1002/btpr.416. Biotechnol Prog. 2010. PMID: 20730758 Free PMC article.
-
Engineering Adhesion of the Probiotic Strain Escherichia coli Nissle to the Fungal Pathogen Candida albicans.ACS Synth Biol. 2024 Dec 20;13(12):4027-4039. doi: 10.1021/acssynbio.4c00466. Epub 2024 Sep 12. ACS Synth Biol. 2024. PMID: 39265099 Free PMC article.
-
Nisin production in a chitin-including continuous fermentation system with Lactococcus lactis displaying a cell wall chitin-binding domain.J Ind Microbiol Biotechnol. 2014 Mar;41(3):535-43. doi: 10.1007/s10295-013-1388-x. Epub 2013 Dec 17. J Ind Microbiol Biotechnol. 2014. PMID: 24342966
References
-
- Bickerstaff, G. F. (ed.). 1997. Methods in biotechnology, vol. 1. Immobilization of enzymes and cells, p. 1-11. Humana Press Inc., Totowa, N.J.
-
- Chao, Y. P., C. J. Chiang, and W. B. Hung. 2002. Stringent regulation and high-level expression of heterologous genes in Escherichia coli using T7 expression system controllable by the araBAD promoter. Biotechnol. Prog. 18:394-400. - PubMed
-
- Chao, Y. P., C. J. Chiang, T. E. Lo, and H. Fu. 2000. Overproduction of D-hydantoinase and carbamoylase in a soluble form in Escherichia coli. Appl. Microbiol. Biotechnol. 54:348-353. - PubMed
-
- Chong, S., F. B. Mersha, D. G. Comb, M. E. Scott, D. Landry, L. M. Vence, F. B. Perler, J. Benner, R. B. Kucera, C. A. Hirvonen, J. J. Pelletier, H. Paulus, and M. Q. Xu. 1997. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene 192:271-281. - PubMed
-
- Daugherty, P. S., M. J. Olsen, B. L. Iverson, and G. Georgiou. 1999. Development of an optimized expression system for the screening of antibody libraries displayed on the Escherichia coli surface. Protein Eng. 12:613-621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

