Autoinducer 2 affects biofilm formation by Bacillus cereus
- PMID: 16391139
- PMCID: PMC1352198
- DOI: 10.1128/AEM.72.1.937-941.2006
Autoinducer 2 affects biofilm formation by Bacillus cereus
Abstract
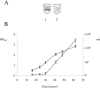
Cell-free supernatants from growing Bacillus cereus strain ATCC 10987 induced luminescence in a Photorhabdus luminescens DeltaluxS mutant, indicating the production of functional autoinducer 2 (AI-2). The exogenous addition of in vitro synthesized AI-2 had an inhibitory effect on biofilm formation by B. cereus and promoted release of the cells from a preformed biofilm.
Figures

References
-
- Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. - PubMed
-
- Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. - PubMed
-
- Hamon, M. A., and B. A. Lazazzera. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42:1199-1209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

