Chameau HAT and DRpd3 HDAC function as antagonistic cofactors of JNK/AP-1-dependent transcription during Drosophila metamorphosis
- PMID: 16391236
- PMCID: PMC1356104
- DOI: 10.1101/gad.359506
Chameau HAT and DRpd3 HDAC function as antagonistic cofactors of JNK/AP-1-dependent transcription during Drosophila metamorphosis
Abstract
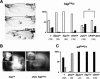
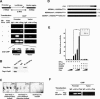
Gene regulation by AP-1 transcription factors in response to Jun N-terminal kinase (JNK) signaling controls essential cellular processes during development and in pathological situations. Here, we report genetic and molecular evidence that the histone acetyltransferase (HAT) Chameau and the histone deacetylase DRpd3 act as antagonistic cofactors of DJun and DFos to modulate JNK-dependent transcription during thorax metamorphosis and JNK-induced apoptosis in Drosophila. We demonstrate in cultured cells that DFos phosphorylation mediated by JNK signaling plays a central role in coordinating the dynamics of Chameau and DRpd3 recruitment and function at AP-1-responsive promoters. Activating the pathway stimulates the HAT function of Chameau, promoting histone H4 acetylation and target gene transcription. Conversely, in response to JNK signaling inactivation, DRpd3 is recruited and suppresses histone acetylation and transcription. This study establishes a direct link among JNK signaling, DFos phosphorylation, chromatin modification, and AP-1-dependent transcription and its importance in a developing organism.
Figures





Similar articles
-
Regulation of NuA4 histone acetyltransferase activity in transcription and DNA repair by phosphorylation of histone H4.Mol Cell Biol. 2005 Sep;25(18):8179-90. doi: 10.1128/MCB.25.18.8179-8190.2005. Mol Cell Biol. 2005. PMID: 16135807 Free PMC article.
-
Enhancer of Acetyltransferase Chameau (EAChm) Is a Novel Transcriptional Co-Activator.PLoS One. 2015 Nov 10;10(11):e0142305. doi: 10.1371/journal.pone.0142305. eCollection 2015. PLoS One. 2015. PMID: 26555228 Free PMC article.
-
Drosophila Ebi mediates Snail-dependent transcriptional repression through HDAC3-induced histone deacetylation.EMBO J. 2008 Mar 19;27(6):898-909. doi: 10.1038/emboj.2008.26. Epub 2008 Feb 28. EMBO J. 2008. PMID: 18309295 Free PMC article.
-
Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression.Biochem Pharmacol. 2004 Sep 15;68(6):1255-67. doi: 10.1016/j.bcp.2004.05.042. Biochem Pharmacol. 2004. PMID: 15313424 Review.
-
Histone acetylation and methylation: combinatorial players for transcriptional regulation.Subcell Biochem. 2007;41:351-69. Subcell Biochem. 2007. PMID: 17484136 Review.
Cited by
-
Predicting gene regulatory interactions based on spatial gene expression data and deep learning.PLoS Comput Biol. 2019 Sep 17;15(9):e1007324. doi: 10.1371/journal.pcbi.1007324. eCollection 2019 Sep. PLoS Comput Biol. 2019. PMID: 31527870 Free PMC article.
-
LSD1: more than demethylation of histone lysine residues.Exp Mol Med. 2020 Dec;52(12):1936-1947. doi: 10.1038/s12276-020-00542-2. Epub 2020 Dec 14. Exp Mol Med. 2020. PMID: 33318631 Free PMC article. Review.
-
Heterochromatin formation in Drosophila requires genome-wide histone deacetylation in cleavage chromatin before mid-blastula transition in early embryogenesis.Chromosoma. 2020 Mar;129(1):83-98. doi: 10.1007/s00412-020-00732-x. Epub 2020 Jan 16. Chromosoma. 2020. PMID: 31950239 Free PMC article.
-
Multiple signals converge on a differentiation MAPK pathway.PLoS Genet. 2010 Mar 19;6(3):e1000883. doi: 10.1371/journal.pgen.1000883. PLoS Genet. 2010. PMID: 20333241 Free PMC article.
-
Human T-cell leukemia virus type-1-encoded protein HBZ represses p53 function by inhibiting the acetyltransferase activity of p300/CBP and HBO1.Oncotarget. 2016 Jan 12;7(2):1687-706. doi: 10.18632/oncotarget.6424. Oncotarget. 2016. PMID: 26625199 Free PMC article.
References
-
- Adachi-Yamada T., Fujimura-Kamada, K., Nishida, Y., and Matsumoto, K. 1999. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400: 166–169. - PubMed
-
- Agalioti T., Chen, G., and Thanos, D. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111: 381–392. - PubMed
-
- Aggarwal B.D. and Calvi, B.R. 2004. Chromatin regulates origin activity in Drosophila follicle cells. Nature 430: 372–376. - PubMed
-
- Agnes F., Suzanne, M., and Noselli, S. 1999. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development 126: 5453–5462. - PubMed
-
- Akhtar A. and Becker, P.B. 2000. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5: 367–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
