The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling
- PMID: 16391536
- PMCID: PMC1369233
- DOI: 10.1038/sj.embor.7400598
The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling
Abstract
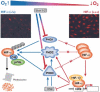
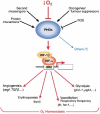
Metazoans rapidly respond to changes in oxygen availability by regulating gene expression. The transcription factor hypoxia-inducible-factor (HIF), which controls the expression of several genes, 'senses' the oxygen concentration indirectly through the hydroxylation of two proline residues that earmarks the HIF-alpha subunits for proteasomal degradation. We review the expression, regulation and function of the HIF prolyl hydroxylases or prolyl hydroxylases domain proteins, which are genuine oxygen sensors.
Figures
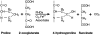

References
-
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465 - PubMed
-
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC (2004) Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem 92: 491–501 - PubMed
-
- Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL (2005) OS-9 interacts with hypoxia-inducible factor 1α and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1α. Mol Cell 17: 503–512 - PubMed
-
- Berra E, Richard DE, Gothie E, Pouyssegur J (2001a) HIF-1-dependent transcriptional activity is required for oxygen-mediated HIF-1α degradation. FEBS Lett 491: 85–90 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

