Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure
- PMID: 16391839
- PMCID: PMC2245862
Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure
Abstract
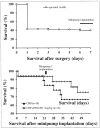
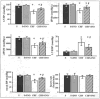
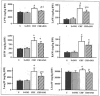
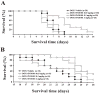
Overactivation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP) contributes to the development of cell dysfunction and tissue injury in various pathophysiological conditions associated with oxidative and nitrosative stress, including myocardial reperfusion injury, heart transplantation, diabetic cardiomyopathy and chronic heart failure. In recent studies, we have demonstrated the beneficial effects of a novel ultrapotent PARP inhibitor, INO-1001, on cardiac and endothelial dysfunction and remodeling in rat model of advanced aging-associated chronic heart failure and in a mouse model of heart failure induced by aortic banding. In the current study, we have investigated the effect of INO-1001 on the development of heart failure induced by permanent ligation of the left anterior descending coronary artery, heart failure induced by doxorubicin and acute myocardial dysfunction induced by bacterial endotoxin. In the coronary ligation model, a significantly depressed left ventricular performance and impaired vascular relaxation of aortic rings were found, and PARP inhibition significantly improved both cardiac function and vascular relaxation. In the doxorubicin model, a single injection of doxorubicin induced high mortality and a significant decrease in left ventricular systolic pressure, +dP/dt, -dP/dt, stroke volume, stroke work, ejection fraction and cardiac output. Treatment with the PARP inhibitor reduced doxorubicin-induced mortality and markedly improved cardiac function. PARP inhibition did not interfere with doxorubicin's antitumor effect. In the endotoxin model of cardiac dysfunction, PARP inhibition attenuated the suppression of myocardial contractility elicited by endotoxin. The current data strengthen the view that PARP inhibition may represent an effective approach for the experimental therapy of various forms of acute and chronic heart failure.
Figures





Similar articles
-
Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure.J Am Coll Cardiol. 2002 Sep 4;40(5):1006-16. doi: 10.1016/s0735-1097(02)02062-4. J Am Coll Cardiol. 2002. PMID: 12225730
-
Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction.Exp Gerontol. 2007 Jul;42(7):676-85. doi: 10.1016/j.exger.2007.01.013. Epub 2007 Feb 20. Exp Gerontol. 2007. PMID: 17383839 Free PMC article.
-
A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging.J Pharmacol Exp Ther. 2004 Nov;311(2):485-91. doi: 10.1124/jpet.104.069658. Epub 2004 Jun 22. J Pharmacol Exp Ther. 2004. PMID: 15213249 Free PMC article.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors.Cardiovasc Drug Rev. 2007 Fall;25(3):235-60. doi: 10.1111/j.1527-3466.2007.00018.x. Cardiovasc Drug Rev. 2007. PMID: 17919258 Free PMC article. Review.
Cited by
-
The clinically active PARP inhibitor AG014699 ameliorates cardiotoxicity but does not enhance the efficacy of doxorubicin, despite improving tumor perfusion and radiation response in mice.Mol Cancer Ther. 2011 Dec;10(12):2320-9. doi: 10.1158/1535-7163.MCT-11-0356. Epub 2011 Sep 16. Mol Cancer Ther. 2011. PMID: 21926192 Free PMC article.
-
Salvage of nicotinamide adenine dinucleotide plays a critical role in the bioenergetic recovery of post-hypoxic cardiomyocytes.Br J Pharmacol. 2015 Oct;172(20):4817-32. doi: 10.1111/bph.13252. Epub 2015 Oct 14. Br J Pharmacol. 2015. PMID: 26218637 Free PMC article.
-
Drug-induced mitochondrial dysfunction and cardiotoxicity.Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1453-67. doi: 10.1152/ajpheart.00554.2015. Epub 2015 Sep 18. Am J Physiol Heart Circ Physiol. 2015. PMID: 26386112 Free PMC article. Review.
-
The tell-tale heart: molecular and cellular responses to childhood anthracycline exposure.Am J Physiol Heart Circ Physiol. 2014 Nov 15;307(10):H1379-89. doi: 10.1152/ajpheart.00099.2014. Epub 2014 Sep 12. Am J Physiol Heart Circ Physiol. 2014. PMID: 25217655 Free PMC article. Review.
-
NAD+ enhancers as therapeutic agents in the cardiorenal axis.Cell Commun Signal. 2024 Nov 8;22(1):537. doi: 10.1186/s12964-024-01903-4. Cell Commun Signal. 2024. PMID: 39516787 Free PMC article. Review.
References
-
- Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(ADP-ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. - PubMed
-
- Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

