Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation
- PMID: 16393655
- PMCID: PMC1332653
- DOI: 10.1289/ehp.8286
Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation
Abstract
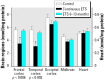
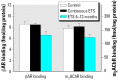
Perinatal environmental tobacco smoke (ETS) exposure in humans elicits neurobehavioral deficits. We exposed rhesus monkeys to ETS during gestation and through 13 months postnatally, or postnatally only (6-13 months). At the conclusion of exposure, we examined cerebrocortical regions and the midbrain for cell damage markers and lipid peroxidation. For perinatal ETS, two archetypal patterns were seen in the various regions, one characterized by cell loss (reduced DNA concentration) and corresponding increases in cell size (increased protein/DNA ratio), and a second pattern suggesting replacement of larger neuronal cells with smaller and more numerous glia (increased DNA concentration, decreased protein/DNA ratio). The membrane/total protein ratio, a biomarker of neurite formation, also indicated potential damage to neuronal projections, accompanied by reactive sprouting. When ETS exposure was restricted to the postnatal period, the effects were similar in regional selectivity, direction, and magnitude. These patterns resemble the effects of prenatal nicotine exposure in rodent and primate models. Surprisingly, perinatal ETS exposure reduced the level of lipid peroxidation as assessed by the concentration of thiobarbituric acid reactive species, whereas postnatal ETS did not. The heart, a tissue that, like the brain, has high oxygen demand, displayed a similar but earlier decrease (2-3 months) in lipid peroxidation in the perinatal exposure model, whereas values were reduced at 13 months with the postnatal exposure paradigm. Our results provide a mechanistic connection between perinatal ETS exposure and neurobehavioral anomalies, reinforce the role of nicotine in these effects, and buttress the importance of restricting or eliminating ETS exposure in young children.
Figures

Similar articles
-
Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain.Brain Res Dev Brain Res. 2002 Feb 28;133(2):175-9. doi: 10.1016/s0165-3806(02)00281-x. Brain Res Dev Brain Res. 2002. PMID: 11882347
-
Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke.Brain Res. 2006 Sep 21;1111(1):30-5. doi: 10.1016/j.brainres.2006.06.094. Epub 2006 Jul 31. Brain Res. 2006. PMID: 16876770
-
Effects of environmental tobacco smoke exposure on pulmonary immune response in infant monkeys.J Allergy Clin Immunol. 2008 Aug;122(2):400-6, 406.e1-5. doi: 10.1016/j.jaci.2008.04.011. Epub 2008 May 27. J Allergy Clin Immunol. 2008. PMID: 18502491
-
A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures.Neurotoxicology. 2005 Aug;26(4):573-87. doi: 10.1016/j.neuro.2004.07.007. Neurotoxicology. 2005. PMID: 16112323 Review.
-
The association between prenatal exposure to environmental tobacco smoke and childhood obesity: a systematic review.JBI Database System Rev Implement Rep. 2018 Aug;16(8):1643-1662. doi: 10.11124/JBISRIR-2017-003558. JBI Database System Rev Implement Rep. 2018. PMID: 30113549
Cited by
-
Is There a Critical Period for the Developmental Neurotoxicity of Low-Level Tobacco Smoke Exposure?Toxicol Sci. 2017 Jan;155(1):75-84. doi: 10.1093/toxsci/kfw180. Epub 2016 Sep 14. Toxicol Sci. 2017. PMID: 27633979 Free PMC article.
-
Cardiovascular Consequences of Childhood Secondhand Tobacco Smoke Exposure: Prevailing Evidence, Burden, and Racial and Socioeconomic Disparities: A Scientific Statement From the American Heart Association.Circulation. 2016 Oct 18;134(16):e336-e359. doi: 10.1161/CIR.0000000000000443. Epub 2016 Sep 12. Circulation. 2016. PMID: 27619923 Free PMC article. Review.
-
Prenatal cigarette exposure and infant learning stimulation as predictors of cognitive control in childhood.Dev Sci. 2011 Jul;14(4):881-91. doi: 10.1111/j.1467-7687.2011.01038.x. Epub 2011 Mar 23. Dev Sci. 2011. PMID: 21676107 Free PMC article.
-
Perinatal nicotine-induced neurotoxicity and behavioral alterations in newborn mice are associated with oxidative stress, inflammation and downregulated Nrf2/HO-1 signaling: protective role of Anethum graveolens.J Mol Histol. 2025 Jun 2;56(3):179. doi: 10.1007/s10735-025-10457-9. J Mol Histol. 2025. PMID: 40455124
-
Prenatal environmental exposures and brain development: studies with baboons and other nonhuman primates.Adv Drug Alcohol Res. 2025 Aug 11;5:14858. doi: 10.3389/adar.2025.14858. eCollection 2025. Adv Drug Alcohol Res. 2025. PMID: 40862205 Free PMC article. Review.
References
-
- Bell JM, Whitmore WL, Queen KL, Orband-Miller L, Slotkin TA. Biochemical determinants of growth sparing during neonatal nutritional deprivation or enhancement: ornithine decarboxylase, polyamines, and macromolecules in brain regions and heart. Pediatr Res. 1987;22:599–604. - PubMed
-
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–839. - PubMed
-
- Eliopoulos C, Klein J, Chitayat D, Greenwald M, Koren G. Nicotine and cotinine in maternal and neonatal hair as markers of gestational smoking. Clin Invest Med. 1996;19:231–242. - PubMed
-
- Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. 2. effect on neurodevelopment at age 5 years. Am J Epidemiol. 1995;142:S19–S29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

