Inducible model for beta-six-mediated site-specific recombination in mammalian cells
- PMID: 16394020
- PMCID: PMC1325017
- DOI: 10.1093/nar/gnj001
Inducible model for beta-six-mediated site-specific recombination in mammalian cells
Abstract
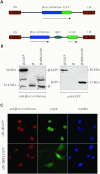
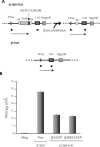
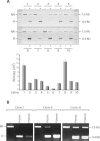
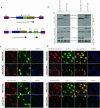
The prokaryotic beta recombinase catalyzes site-specific recombination between two directly oriented minimal six sites in chromatin-integrated substrates. Here, we demonstrate that an enhanced green fluorescent protein (EGFP)-fused version of beta recombinase (beta-EGFP) is fully active, retaining most specific activity. It is used to develop a recombination-dependent activatable gene expression (RAGE) system based on the androgen receptor (AR) ligand-binding domain (LBD). Two hybrid molecules, a direct fusion of the LBD-AR to the C-terminus of beta recombinase (beta-AR) and a triple fusion of beta-EGFP to the same ligand-binding domain (beta-EGFP-AR), were engineered and their subcellular behavior, stability and catalytic activity were evaluated. Both chimeric beta recombinase proteins showed in vivo inducible recombinogenic activity dependent on addition of an androgen receptor agonist, although the beta-AR fusion protein demonstrated more accurate ligand-dependent translocation from cytoplasm to nucleus.
Figures

Similar articles
-
New insights into host factor requirements for prokaryotic beta-recombinase-mediated reactions in mammalian cells.J Biol Chem. 2001 May 11;276(19):16257-64. doi: 10.1074/jbc.M011725200. Epub 2001 Jan 30. J Biol Chem. 2001. PMID: 11278972
-
Functional in vivo interaction between the amino-terminal, transactivation domain and the ligand binding domain of the androgen receptor.Biochemistry. 1997 Feb 4;36(5):1052-64. doi: 10.1021/bi961775g. Biochemistry. 1997. PMID: 9033395
-
Induction of cre recombinase activity using modified androgen receptor ligand binding domains: a sensitive assay for ligand-receptor interactions.Nucleic Acids Res. 2003 Aug 1;31(15):e86. doi: 10.1093/nar/gng087. Nucleic Acids Res. 2003. PMID: 12888538 Free PMC article.
-
Mammalian genome targeting using site-specific recombinases.Front Biosci. 2006 Jan 1;11:1108-36. doi: 10.2741/1867. Front Biosci. 2006. PMID: 16146801 Review.
-
Genome engineering using site-specific recombinases.Cloning Stem Cells. 2002;4(1):65-80. doi: 10.1089/153623002753632066. Cloning Stem Cells. 2002. PMID: 12006158 Review.
Cited by
-
Functionality of the beta/six site-specific recombination system in tobacco and Arabidopsis: a novel tool for genetic engineering of plant genomes.Plant Mol Biol. 2007 Mar;63(4):545-56. doi: 10.1007/s11103-006-9108-9. Plant Mol Biol. 2007. PMID: 17131098
-
Expanding the zinc-finger recombinase repertoire: directed evolution and mutational analysis of serine recombinase specificity determinants.Nucleic Acids Res. 2014 Apr;42(7):4755-66. doi: 10.1093/nar/gkt1389. Epub 2014 Jan 21. Nucleic Acids Res. 2014. PMID: 24452803 Free PMC article.
References
-
- Metzger D., Feil R. Engineering the mouse genome by site-specific recombination. Curr. Opin. Biotechnol. 1999;10:470–476. - PubMed
-
- Sauer B. Site-specific recombination: developments and applications. Curr. Opin. Biotechnol. 1994;5:521–527. - PubMed
-
- Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. - PubMed
-
- Rossant J., McMahon A. ‘Cre’-ating mouse mutants- a meeting review on conditional mouse genetics. Genes Dev. 1999;13:142–145. - PubMed
-
- Agah R., Frenkel P.A., French B.A., Michael L.H., Overbeek P.A., Schneider M.D. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 1997;100:169–179. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

