Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans
- PMID: 16394102
- PMCID: PMC1382311
- DOI: 10.1091/mbc.e05-06-0533
Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans
Abstract
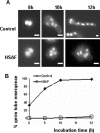
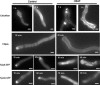
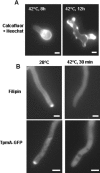
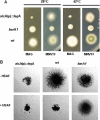
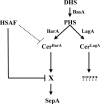
In filamentous fungi, the stabilization of a polarity axis is likely to be a pivotal event underlying the emergence of a germ tube from a germinating spore. Recent results implicate the polarisome in this process and also suggest that it requires localized membrane organization. Here, we employ a chemical genetic approach to demonstrate that ceramide synthesis is necessary for the formation of a stable polarity axis in the model fungus Aspergillus nidulans. We demonstrate that a novel compound (HSAF) produced by a bacterial biocontrol agent disrupts polarized growth and leads to loss of membrane organization and formin localization at hyphal tips. We show that BarA, a putative acyl-CoA-dependent ceramide synthase that is unique to filamentous fungi mediates the effects of HSAF. Moreover, A. nidulans possesses a second likely ceramide synthase that is essential and also regulates hyphal morphogenesis. Our results suggest that filamentous fungi possess distinct pools of ceramide that make independent contributions to polarized hyphal growth, perhaps through the formation of specialized lipid microdomains that regulate organization of the cytoskeleton.
Figures




References
-
- Bartnicki-Garcia, S. (2002). Hyphal tip growth: outstanding questions. In: Molecular Biology of Fungal Development, ed. H. D. Osiewacz, New York: Marcel Dekker, 29–58.
-
- Bartnicki-Garcia, S., and Lippman, E. (1969). Fungal morphogenesis: cell wall construction in Mucor rouxii. Science 165, 302–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

