Isolation and purification of clemastanin B and indigoticoside A from Radix Isatidis by high-speed counter-current chromatography
- PMID: 16395796
- PMCID: PMC7094637
- DOI: 10.1016/j.chroma.2005.07.072
Isolation and purification of clemastanin B and indigoticoside A from Radix Isatidis by high-speed counter-current chromatography
Abstract
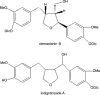
A preparative high-speed counter-current chromatography (HSCCC) with a two-phase solvent system composed of ethyl acetate-n-butanol-water (2:7:9, v/v/v) was successfully performed to isolate and separate clemastanin B and indigoticoside A from the plant of Radix Isatidis, a traditional Chinese medicine. A total of 59.2 mg clemastanin B and 66.1 mg indigoticoside A with purities of 94.6% and 99.0% determined by high performance liquid chromatography (HPLC) were obtained in one-step elution from 250 mg crude extract, which contained clemastanin B 24.8% and indigoticoside A 28.4%, and the recoveries of clemastanin B and indigoticoside A were 90.3% and 92.2%, respectively. The chemical structure was identified by IR, MS, 1H NMR and 13C NMR.
Figures


Similar articles
-
Preparative isolation and purification of indigo and indirubin from Folium isatidis by high-speed counter-current chromatography.Phytochem Anal. 2012 Nov-Dec;23(6):637-41. doi: 10.1002/pca.2366. Epub 2012 May 3. Phytochem Anal. 2012. PMID: 22553192
-
On-line purity monitoring in high-speed counter-current chromatography: application of HSCCC-HPLC-DAD for the preparation of 5-HMF, neomangiferin and mangiferin from Anemarrhena asphodeloides Bunge.J Pharm Biomed Anal. 2007 May 9;44(1):96-100. doi: 10.1016/j.jpba.2007.01.050. Epub 2007 Feb 4. J Pharm Biomed Anal. 2007. PMID: 17349768
-
Preparative separation of four major bufadienolides from the Chinese traditional medicine, Chansu, using high-speed counter-current chromatography.Nat Prod Commun. 2010 Jul;5(7):1031-4. Nat Prod Commun. 2010. PMID: 20734934
-
Preparative isolation and purification of four compounds from the Chinese medicinal herb rhizoma Anemarrhenae by high-speed counter-current chromatography.J Chromatogr A. 2006 Feb 3;1104(1-2):69-74. doi: 10.1016/j.chroma.2005.11.046. Epub 2005 Dec 20. J Chromatogr A. 2006. PMID: 16364341
-
Alternative Extraction and Downstream Purification Processes for Anthocyanins.Molecules. 2022 Jan 7;27(2):368. doi: 10.3390/molecules27020368. Molecules. 2022. PMID: 35056685 Free PMC article. Review.
Cited by
-
Integrative analyses of targeted metabolome and transcriptome of Isatidis Radix autotetraploids highlighted key polyploidization-responsive regulators.BMC Genomics. 2021 Sep 17;22(1):670. doi: 10.1186/s12864-021-07980-w. BMC Genomics. 2021. PMID: 34535080 Free PMC article.
-
[Antiviral activities against influenza virus (FM1) of bioactive fractions and representative compounds extracted from Banlangen (Radix Isatidis)].J Tradit Chin Med. 2016 Jun;36(3):369-76. doi: 10.1016/s0254-6272(16)30051-6. J Tradit Chin Med. 2016. PMID: 27468553 Free PMC article.
-
SEPARATION AND PURIFICATION OF TWO MINOR COMPOUNDS FROM RADIX ISATIDIS BY INTEGRATIVE MPLC AND HSCCC WITH PREPARATIVE HPLC.J Liq Chromatogr Relat Technol. 2015 Jan 1;38(5):647-653. doi: 10.1080/10826076.2014.936606. J Liq Chromatogr Relat Technol. 2015. PMID: 25745338 Free PMC article.
-
Research progress on antiviral constituents in traditional Chinese medicines and their mechanisms of action.Pharm Biol. 2022 Dec;60(1):1063-1076. doi: 10.1080/13880209.2022.2074053. Pharm Biol. 2022. PMID: 35634712 Free PMC article.
-
Modes of Antiviral Action of Chemical Portions and Constituents from Woad Root Extract against Influenza Virus A FM1.Evid Based Complement Alternat Med. 2016;2016:2537294. doi: 10.1155/2016/2537294. Epub 2016 Feb 18. Evid Based Complement Alternat Med. 2016. PMID: 26989425 Free PMC article.
References
-
- Gao X.Z. Res. Trad. Chin. Med. 2001;17:57.
-
- Li S., Chen W.S., Qiao C.Z., Zheng S.Q. J. Second Mil. Med. Univ. 2000;21:204.
-
- Wang Y., Qiao C.Z., Liu S., Zhang H.M. China J. Chin. Mater. Med. 2000;25:327. - PubMed
-
- Xu H., Fang J.G., Liu Y.H. Chin. Tradit. Herbal Drugs. 2003;34:10.
-
- Shang H.C., Gao X.M., Chen J. Tianjin J. Tradit. Chin. Med. 2003;20:97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

